题目内容
【题目】NaClO2的漂白能力是漂白粉的4~5倍, NaClO2广泛用于造纸工业、污水处理等。工业上生产NaClO2的工艺流程如下:

(1)ClO2发生器中的反应为:2NaClO3+SO2+H2SO4===2ClO2+2NaHSO4。实际工业生产中,可用硫黄、浓硫酸代替原料中的SO2,其原因为__________________(用化学方程式表示)。
(2)反应结束后,向ClO2发生器中通入一定量空气的目的:________________________。
(3)吸收器中生成NaClO2的离子反应方程式为________________________________。
(4)某化学兴趣小组用如下图所示装置制备SO2并探究SO2与Na2O2的反应:
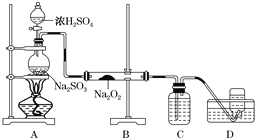
①盛放浓H2SO4仪器名称为____________。
②D中收集到的气体可使带余烬的木条复燃,B中发生的反应可能为__________________、Na2O2+SO2===Na2SO4。
【答案】 S+2H2SO4(浓)![]() 3SO2↑+ 2H2O 驱赶出ClO2,确保其被充分吸收 2ClO2+2OH-+H2O2===2ClO
3SO2↑+ 2H2O 驱赶出ClO2,确保其被充分吸收 2ClO2+2OH-+H2O2===2ClO![]() +O2+2H2O 分液漏斗 2Na2O2+2SO2===2Na2SO3+O2
+O2+2H2O 分液漏斗 2Na2O2+2SO2===2Na2SO3+O2
【解析】(1)ClO2发生器中的反应为氯酸钠与二氧化硫在浓硫酸作用下发生氧化还原反应,而硫磺、浓硫酸也可以生成二氧化硫,反应的方程式为S+2H2SO4(浓)![]() 3SO2↑+2H2O,所以可用硫磺、浓硫酸代替原料中的SO2,故答案为:S+2H2SO4(浓)
3SO2↑+2H2O,所以可用硫磺、浓硫酸代替原料中的SO2,故答案为:S+2H2SO4(浓)![]() 3SO2↑+2H2O;
3SO2↑+2H2O;
(2)反应结束后,发生器中仍有少量ClO2,用空气可以将其排出,确保其被充分吸收,故答案为:驱赶出ClO2,确保其被充分吸收;
(3)吸收器中双氧水与ClO2在碱性条件下发生氧化还原反应生成NaClO2,反应的离子方程式为2ClO2+2OH-+H2O2=2ClO2-+O2+2H2O,故答案为:2ClO2+2OH-+H2O2=2ClO2-+O2+2H2O;
(4)①根据装置图,盛放浓H2SO4仪器为分液漏斗,故答案为:分液漏斗;
②D中收集到的气体可使带余烬的木条复燃,说明有氧气产生,所以B中发生的反应可能为二氧化硫与过氧化钠反应生成亚硫酸钠和氧气,反应的方程式为2Na2O2+2SO2=2Na2SO3+O2,故答案为:2Na2O2+2SO2=2Na2SO3+O2。


