��Ŀ����
11��ij��ɫ��Һ��Mg2+��Al3+��Cl-��NH4+��SO42-�е�������������ɣ������²������ʵ�飺��1��ȡԭ��Һ�������Թ��У����Թ��в��ϼ�������������Һ���������а�ɫ�������ɶ�������ȫ��ʧ��˵��ԭ��Һ��һ�����е�������Al3+������Ӧ���ӷ��ţ���ͬ����
��2��ȡʵ��ٺ����Һ����һ�Թ��У����ȣ���ʪ��ĺ�ɫʯ����ֽ�����Թܿڣ������ֽ������˵��ԭ��Һ��һ�����е�������NH4+��������ӷ���ʽΪ��NH4++OH-=$\frac{\underline{\;\;��\;\;}}{\;}$NH3��+H2O
��3��ȡԭ��Һ������������ʹ��Һ�ữ���ټ����Ȼ�����Һ��������ɫ������˵��ԭ��Һ��һ�����е�������SO42-��������ӷ���ʽΪ��Ba2++SO42-=BaSO4��
��4��ȡʵ��۵���Һ����������������Һ��������ɫ�������ܷ�˵��ԭ��Һ��һ�����е�Cl-���ӷ��ǻ����Ҫ���Cl-�����Ƿ���ڣ���ȷ�IJ����ǣ���ǰһ�����ǣ���һ�����ȡԭ��Һ�������Թ��У��������ữ���ٵμ���������Һ�����а�ɫ������֤�������Ӵ��ڣ����������ӣ�
���� ��1��ȡԭ��Һ�������Թ��У����Թ��в��ϼ�������������Һ���������а�ɫ�������ɶ�������ȫ��ʧ��˵��ԭ��Һ��һ������Al3+��һ������Mg2+��
��2��ȡʵ��ٺ����Һ����һ�Թ��У����ȣ���ʪ��ĺ�ɫʯ����ֽ�����Թܿڣ������ֽ������Ӧ���ɰ�����˵������NH4+��
��3��ȡԭ��Һ������������ʹ��Һ�ữ���ټ����Ȼ�����Һ��������ɫ������˵������SO42-��
��4��ȡʵ��۵���Һ����������������������Һ��������ɫ������˵������AgCl��������˵��ԭ��Һ����Cl-����ʵ�飨3�������Ȼ�����
��� �⣺��1��ȡԭ��Һ�������Թ��У����Թ��в��ϼ�������������Һ���������а�ɫ�������ɶ�������ȫ��ʧ��˵��ԭ��Һ��һ������Al3+��
�ʴ�Ϊ��Al3+��
��2��ȡʵ��ٺ����Һ����һ�Թ��У����ȣ���ʪ��ĺ�ɫʯ����ֽ�����Թܿڣ������ֽ������Ӧ���ɰ�����˵������NH4+����Ӧ�����ӷ���ʽΪ��NH4++OH-=$\frac{\underline{\;\;��\;\;}}{\;}$NH3��+H2O��
�ʴ�Ϊ��NH4+��NH4++OH-=$\frac{\underline{\;\;��\;\;}}{\;}$NH3��+H2O��
��3��ȡԭ��Һ������������ʹ��Һ�ữ���ټ����Ȼ�����Һ��������ɫ������˵������SO42-����Ӧ�����ӷ���ʽΪ��Ba2++SO42-=BaSO4����
�ʴ�Ϊ��SO42-��Ba2++SO42-=BaSO4����
��4��ȡʵ��۵���Һ����������������������Һ��������ɫ������˵������AgCl��������˵��ԭ��Һ����Cl-����ʵ�飨3�������Ȼ�������Ҫ���Cl-�����Ƿ���ڣ���ȷ�IJ�����ȡԭ��Һ�������Թ��У��������ữ���ٵμ���������Һ�����а�ɫ������֤�������Ӵ��ڣ����������ӣ�
�ʴ�Ϊ����ȡԭ��Һ�������Թ��У��������ữ���ٵμ���������Һ�����а�ɫ������֤�������Ӵ��ڣ����������ӣ�
���� ���⿼�鳣�����ӵļ��鷽����Ϊ��Ƶ���㣬��Ŀ�Ѷ��еȣ����ճ�������֮��ķ�Ӧ�����Ӽ����Ϊ�ƶϵĹؼ������ط������ƶ��������ۺϿ��飮

| ������ ���� ���� | ��A | ��A | ��A | ��A | ��A | ��A | ��A | 0�� |
| 2 | A | B | C | |||||
| 3 | D | E | F | G | H | |||
| 4 | I | J |
��2��������������ˮ�����У�������ǿ�Ļ�����Ļ�ѧʽ��HClO4�����������ǿ�Ļ����ﷴӦ�����ӷ���ʽΪH++OH-=H2O��
��3��C��D��ԭ�Ӹ�����1��1�γɵĻ�����ĵ���ʽΪ
 ���û������������Ӻ��ۼ��γɣ�
���û������������Ӻ��ۼ��γɣ���4��ijͬѧ��ͬ����Ԫ��D��E��F��G��H���ʵݱ����ʵ��ʱ���Լ������һ��ʵ�鷽��������¼���й�ʵ��������������еġ�ʵ�鲽�衱�롰ʵ������ǰ��һ���Ƕ�Ӧ��ϵ����
| ʵ�鲽�� | ʵ������ |
| �ٽ�E������ɰֽ��ĥ�����Թ��У���������ˮ������ˮ���ڣ�������Һ�еμӷ�̪��Һ | 1������ˮ���ϣ��۳�С���Ĵ��ζ���������˻˻��������֮��ʧ����Һ��ɺ�ɫ�� |
| �������Ƶ�Na2G��Һ�еμ����Ƶĵ���H��ˮ��Һ | 2���������������Һ���dz��ɫ |
| �۽�һС�����D������з�̪��Һ����ˮ�� | 3�����ҷ�Ӧ��Ѹ�ٲ���������ɫ���壮 |
| �ܽ�����EͶ��ϡ������ | 4����Ӧ��ʼ��ʮ�־��ң�������ɫ���壮 |
| �ݽ�����FͶ��ϡ������ | 5�����ɰ�ɫ��״�������̶�������ʧ |
| ����FH3��Һ�еμ�DOH��Һ������ | 6�����ɵ���ɫ������ |
ʵ�����ݣ�
ʵ��ڡ�ʵ�������Ӧ��ʵ������ֱ�Ϊ6��3������ţ�
ʵ����з�����Ӧ�����ӷ���ʽΪAl3++3OH-=Al��OH��3����Al��OH��3+OH-=AlO2-+2H2O��
ʵ����ۣ�������ͬ����Ԫ��ԭ�ӵ�ʧ���������ݼ����õ�������������
| A�� | ��ӦC��s��+CO2��g���T2CO��g���ġ�H��0����S��0 | |
| B�� | ��ӦH+��aq��+OH-��aq���TH2O��l���ġ�H��0����S��0 | |
| C�� | ��ӦNH3��g��+HCl��g���TNH4Cl��s�� �ڵ��������Է����У���÷�Ӧ��H��0 | |
| D�� | ��ӦCaCO3��s���TCaO��s��+CO2��g�� �����²����Է����У���÷�Ӧ��H��0 |
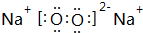
| A�� | �����ӵĽṹʾ��ͼ�� | B�� | ��ϩ�Ľṹ��ʽ��CH2CH2 | ||
| C�� | CH4���ӵ����ģ�ͣ�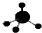 | D�� | -CH3�������ĵ���ʽΪ��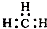 |
| ���� | ұ��ԭ�� | |
| A | Fe | Fe2O3+3CO$\frac{\underline{\;����\;}}{\;}$ 2Fe+3CO2 |
| B | Hg | 2HgO $\frac{\underline{\;\;��\;\;}}{\;}$ 2Hg+O2�� |
| C | Al | 2AlCl3�����ڣ�$\frac{\underline{\;ͨ��\;}}{\;}$ 2Al+3Cl3�� |
| D | Na | 2NaCl�����ڣ�$\frac{\underline{\;ͨ��\;}}{\;}$ 2Na+Cl2�� |
| A�� | A | B�� | B | C�� | C | D�� | D |
2CH3OH��l��+3O2��g���T2CO2��g��+4H2O��l����H=-1452kJ•mol-1
H+��aq��+OH-��aq���TH2O��l����H=-57.3kJ•mol-1
����˵����ȷ���ǣ�������
| A�� | H2��g����ȼ����Ϊ571.6 kJ•mol-1 | |
| B�� | $\frac{1}{2}$H2SO4��aq��+$\frac{1}{2}$Ba��OH��2��aq���T$\frac{1}{2}$BaSO4��s��+H2O��l����H=-57.3 kJ•mol-1 | |
| C�� | ͬ������H2��g����CH3OH��l����ȫȼ�գ�H2��g���ų��������� | |
| D�� | 3H2��g��+CO2��g���TCH3OH��l��+H2O��l����H=+131.4 kJ•mol-1 |
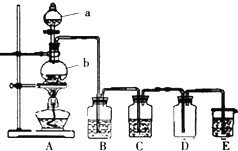
 +Br2$\stackrel{FeBr_{3}}{��}$
+Br2$\stackrel{FeBr_{3}}{��}$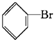 +HBr��
+HBr��