��Ŀ����
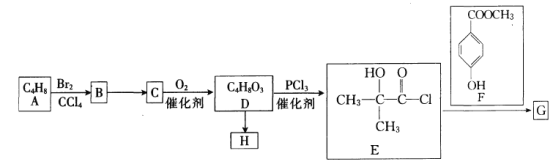
����Ŀ���ԷϾ�п�̵���еĺ��̷ۣ�MnO2��MnO��OH����NH4Cl������ZnCl2��̿�ڡ��������ȣ�Ϊԭ���Ʊ�MnCl2��ʵ���̵������á��乤���������£�
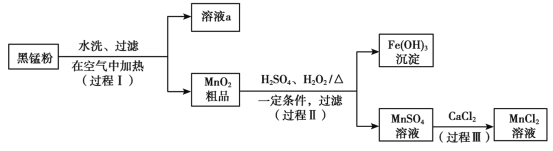
��1�����̢��У��ڿ����г�ּ��Ⱥ��̷۵�Ŀ����Ҫ�dz�ȥ__________��������Ԫ�أ���д����MnԪ�ص����ʷ�����Ӧ�Ļ�ѧ����ʽ____________________________��
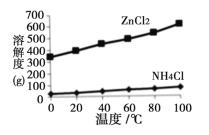
��2����Һa����Ҫ�ɷ�ΪNH4Cl�������������ZnCl2�ȡ�������ͼ��ʾ���ܽ�����ߣ�����Һa��___________��_______________�����˿ɵ�NH4Cl��Ʒ��
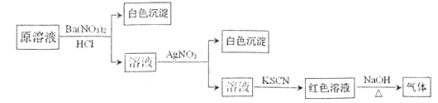
��3������MnSO4��Һ���Ƿ���Fe3����ȡ������Һ������____________�����Լ�������֤����Һ��Fe3��������ȫ��
��4��̽�����̢���MnO2�ܽ������������
��.��MnO2�м���H2O2��Һ�������������ݣ��ټ���ϡH2SO4������δ�����ܽ⡣
��.��MnO2�м���ϡH2SO4������δ�ܽ⣻�ټ���H2O2��Һ�������������ݣ�������ȫ�ܽ⡣
���û�ѧ����ʽ��ʾ����MnO2�ܽ��ԭ��__________________��
�ڽ����Լ�����˳��ͬ��MnO2���ò�ͬ��ԭ��_______________________��
����ʵ��˵�����Լ�����˳��ͬ���������ֵ����ʿ��ܲ�ͬ������Ҳ���ܲ�ͬ��
���𰸡�̿�� 4MnO��OH��+O2=4MnO2+2H2O ����Ũ�� ���½ᾧ KSCN��Һ������� MnO2+H2SO4+H2O2=MnSO4+O2![]() +2H2O ��.MnO2���������ӿ췴Ӧ���ʣ�ֻ���ֽ⣻��.MnO2��������������ϡH2SO4���������̵���������ǿ������ԭΪ������
+2H2O ��.MnO2���������ӿ췴Ӧ���ʣ�ֻ���ֽ⣻��.MnO2��������������ϡH2SO4���������̵���������ǿ������ԭΪ������
��������
��1�����Ⱥ��̷ۣ�̼��������е�������Ӧ���ɶ�����̼��+3�۵������ɶ������̣�����Ԫ�ػ��ϼ۲��䣻
��2�������ܽ�ȵ�ͼ�������ʵ��ܽ�Ȳ��ϴɲ��ýᾧ�ķ�ʽ���룻
��3��Fe3������KSCN��Һ��Ѫ��ɫ��
��4����.��MnO2�м���H2O2��Һ�������������������ӿ췴Ӧ���ʣ���ϡ�����Ӧ����.��MnO2�м���ϡH2SO4���������̵���������ǿ����������ⷴӦ���������̺�������
��1�����Ⱥ��̷ۣ�̼��������е�������Ӧ���ɶ�����̼��+3�۵������ɶ������̣�����Ԫ�ػ��ϼ۲��䣻��Ӧ�к�MnԪ�ػ��ϼ۸ı�ķ���ʽΪ4MnO��OH��+O2=4MnO2+2H2O��
��2�������ܽ�ȵ�ͼ�������ʵ��ܽ�Ȳ��ϴɲ��ýᾧ�ķ�ʽ���룬�����Ϊ����Ũ�������½ᾧ�����ˡ�ϴ�ӡ�����ɵõ�ZnCl2��NH4Cl��Ʒ��
��3��Fe3������KSCN��Һ��Ѫ��ɫ��ȡ������Һ���μ�KSCN��Һ������Һ����죬��֤����Һ��Fe3��������ȫ��
��4����.��MnO2�м���H2O2��Һ�������������������ӿ췴Ӧ���ʣ���ϡ�����Ӧ����.��MnO2�м���ϡH2SO4���������̵���������ǿ����������ⷴӦ���������̺������������ķ���ʽΪMnO2+H2SO4+H2O2=MnSO4+O2![]() +2H2O��
+2H2O��

����Ŀ��������2 L���ݵ������У��ֱ�����ӦA��g��+3B��g��![]() 2C��g����5min���������Ӧ��ƽ��״̬�����м�������A�����ʵ���Ϊ0.4 mol������˵����ȷ���ǣ���
2C��g����5min���������Ӧ��ƽ��״̬�����м�������A�����ʵ���Ϊ0.4 mol������˵����ȷ���ǣ���
��Ӧǰ�����ʵ����ʵ���/mol
A | B | C | |
�� | 1 | 3 | 0 |
�� | 0 | 0 | 2 |
�� | 1.5 | 4.5 | 1 |
A.����5min��C�ķ�Ӧ����Ϊ0.16mol-1��min-1
B.�ﵽƽ��ʱ������A��Ũ���Ǽ��е�2��
C.���з�Ӧ��ƽ�ⳣ�����ڼ��з�Ӧ��ƽ�ⳣ��
D.�ﵽƽ��ʱ���ס�����������C�����ʵ������
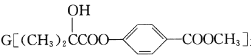
����Ŀ�����ײ���һֱ�������о�����Ҫ���⣬��������Fe�۱�������г�ǿ�Ĵ��ԡ���Ч���Ե����������ʡ�
��.ʵ���Ҳ������ԭ���Ʊ�����Fe����������ͼ��ʾ:

(1)����Fe��ϡ���ᷴӦ�����ӷ���ʽΪ__________________________��
(2)��ν�FeCl2��nH2O���������ˮ�Ƶ���ˮFeCl2_________(�ü�Ҫ��������)��
(3)��������Fe�Ļ�ѧ����ʽΪ__________________��
��.�������ϣ��ڲ�ͬ�¶��£�����Fe����ˮ������Ӧ�Ĺ�����ﲻͬ���¶ȵ���570 ��ʱ����FeO������570 ��ʱ����Fe3O4����ͬѧ����ͼ����ʾװ�ý�������Fe����ˮ������Ӧ��ʵ�飬��ͬѧ��ͼ����ʾ��װ�ý�������Fe����ˮ�����ķ�Ӧ����֤���
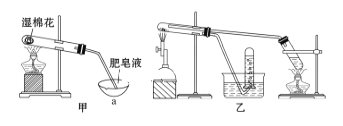
(4)��װ��������Fe����ˮ������Ӧ�Ļ�ѧ����ʽ��____________��
(5)��ͬѧΪ̽��ʵ��������Թ��ڵĹ������ʳɷ֣�����������ʵ��:
ʵ�鲽�� | ʵ����� | ʵ������ |
�� | ����Ӧ��õ��ĺ�ɫ��ĩX(�ٶ�Ϊ���ȵ�)��ȡ������������һ�Թ��У������������ᣬ�� | ��ɫ��ĩ���ܽ⣬��Һ��dz��ɫ�����������ݲ��� |
�� | ��ʵ��ٵõ�����Һ�еμӼ���KSCN��Һ���� | ��Һû�г��ֺ�ɫ |
��������ʵ�飬��ͬѧ��Ϊ�������·�Ӧ�Ĺ������ΪFeO����ͬѧ��Ϊ��ͬѧ�Ľ��۲���ȷ������������________(�ü�Ҫ��������)��
(6)��ͬѧ��ȡ5.60 g Fe�ۣ�����װ�÷�Ӧһ��ʱ���ֹͣ���ȡ����Թ��ڵĹ��������ڸ���������ȴ�Ƶ�����Ϊ6.88 g����ͬѧʵ���Ĺ������������������������Ϊ___(���������λ��Ч����)��
����Ŀ����ҵ�ϳɰ���ӦΪ��N2��g��+3H2��g��![]() 2NH3��g���������о����£�
2NH3��g���������о����£�
��1����֪H��H����Ϊ436kJ��mol��1��N��H����Ϊ391kJ��mol��1��N��N���ļ�����946kJ��mol��1����������Ӧ����H��_________________��
��2��������Ӧ��ƽ�ⳣ��K�ı���ʽΪ____________������Ӧ����ʽ��дΪNH3��g��![]() N2��g��+
N2��g��+![]() H2��g������ƽ�ⳣ��K1��____________________����K��ʾ����
H2��g������ƽ�ⳣ��K1��____________________����K��ʾ����
��3����773Kʱ���ֱ�2mol N2��6mol H2����һ���̶��ݻ�Ϊ1L���ܱ������У����ŷ�Ӧ�Ľ��У�����������n��H2����n��NH3���뷴Ӧʱ��t�Ĺ�ϵ���±���
t/min | 0 | 5 | 10 | 15 | 20 | 25 | 30 |
n��H2��/mol | 6.00 | 4.50 | 3.60 | 3.30 | 3.03 | 3.00 | 3.00 |
n��NH3��/mol | 0 | 1.00 | m | 1.80 | 1.98 | 2.00 | 2.00 |
�ٱ�����m��_______________/span>��15��25min�ڣ�v��N2����_______________��
�ڸ��¶��£�����ͬ�ݻ�����һ������Ͷ���N2��H2��NH3Ũ�Ⱦ�Ϊ3mol��L��1����ʱv��_______v��������������������������������
���ɱ��е�ʵ�����ݼ���õ���Ũ�ȡ�ʱ�����Ĺ�ϵ����ͼ�е����߱�ʾ����ʾc��N2����t��������______________�������������������������������ڴ��¶��£�����ʼ����4mol N2��12mol H2����Ӧ�մﵽƽ��ʱ����ʾc��H2������������Ӧ�ĵ�Ϊ_________________��

��4��Marnellos��Stoukides���õ�ⷨ�ϳɰ���ʵ���˳�ѹ�ϳɺ͵����ĸ�ת���ʡ��÷�����SCY�մɽ�����������SCY�մɾ��и����ӵ����ԣ��������Ǵ���H�����������ĵ缫��ӦΪ____________________��