��Ŀ����
��16�֣�������ҵ�г����ұ�����ķ����Ʊ�����ϩ��
��1����֪ij�¶��£�
��Ӧ�٣�CO2��g�� +H2 ��g����CO��g�� + H2O��g������H�� +41.2 kJ/mol
��Ӧ�ڣ� 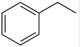 ��g����
��g����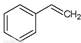 ��g��+H2��g������H=" +117.6" kJ/mol
��g��+H2��g������H=" +117.6" kJ/mol
�ڵĻ�ѧ��Ӧƽ�ⳣ���ֱ�ΪK1��K2��
��д��������̼�����ұ��Ʊ�����ϩ���Ȼ�ѧ��Ӧ����ʽ ���÷�Ӧ�Ļ�ѧƽ�ⳣ��K�� ����K1��K2��ʾ����
��2�����ڷ�Ӧ�٣����º��������£����ܱ������м���2molCO2��2molH2������Ӧ�ﵽƽ�������˵����ȷ���� ��
| A����Ϊ�÷�Ӧ�����ȷ�Ӧ�����������¶ȣ�����Ӧ���������淴Ӧ���ʼ�С�� |
| B������������1molCO2��1mol H2��ƽ��������Ӧ�����ƶ��� |
| C��������ͨ��1mol CO2��ƽ��������Ӧ�����ƶ���CO2��ת�������� |
| D��ѹ�������ƽ�ⲻ�ƶ�����Ӧ��Ͳ����Ũ�ȶ����䣻 |
��4����֪ij�¶��£� Ag2SO4��M��312g/mol�����ܽ��Ϊ0.624g/100g H2O�����¶���Ksp��Ag2SO4���� ������λ��Ч���֣�
��5����ⷨ�Ʊ��������ƣ�Na2FeO4�����ܷ�ӦʽΪ��Fe+2H2O+2OH- �� FeO42-+3H2���������Һѡ��NaOH��Һ���õ������������� (д��ѧʽ) �������ĵ缫��ӦʽΪ�� ��
��1��CO2��g��+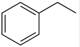 ��g����
��g���� ��g��+ H2O��g������H�� +158.8 kJ/mol����3�֣�
��g��+ H2O��g������H�� +158.8 kJ/mol����3�֣�
K��K1��K����2�֣� ��2��B��2�֣� (3) 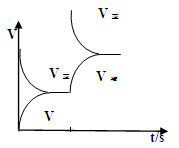 ��2�֣�
��2�֣�
��4��3.2��10-5 ��3�֣� ��5�� Fe Fe-6e+8OH- ��FeO42-+4H2O����2�֣�
���������������1����֪��Ӧ�٣�CO2��g�� +H2 ��g����CO��g�� + H2O��g������H�� +41.2 kJ/mol����Ӧ�ڣ�  ��g����
��g���� ��g��+H2��g������H=" +117.6" kJ/mol������ݸ�˹���ɿ�֪��+�ڼ��õ��Ȼ�ѧ����ʽCO2��g��+
��g��+H2��g������H=" +117.6" kJ/mol������ݸ�˹���ɿ�֪��+�ڼ��õ��Ȼ�ѧ����ʽCO2��g��+ ��g����
��g���� ��g��+ H2O��g�� ��H�� +158.8 kJ/mol����ѧƽ�ⳣ������һ�������£������淴Ӧ�ﵽƽ��״̬ʱ��������Ũ�ȵ���֮���ͷ�Ӧ��Ũ�ȵ���֮���ı�ֵ����÷�Ӧ�Ļ�ѧƽ�ⳣ��
��g��+ H2O��g�� ��H�� +158.8 kJ/mol����ѧƽ�ⳣ������һ�������£������淴Ӧ�ﵽƽ��״̬ʱ��������Ũ�ȵ���֮���ͷ�Ӧ��Ũ�ȵ���֮���ı�ֵ����÷�Ӧ�Ļ�ѧƽ�ⳣ��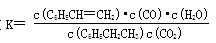
��2��A�������¶ȣ�����Ӧ���������淴Ӧ����Ҳ����A����ȷ��B���¶ȡ�������䣬����������1molCO2��1mol H2����Ӧ��Ũ������ƽ��������Ӧ�����ƶ���B��ȷ��C��������ͨ��1mol CO2����Ӧ��Ũ������ƽ��������Ӧ�����ƶ�����CO2��ת���ʼ�С��C����ȷ��D����Ӧǰ��������䣬ѹ�������ƽ�ⲻ�ƶ�������Ӧ��Ͳ����Ũ�ȶ�����D����ȷ����ѡB��
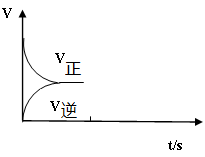
��3���º��������£���Ӧ�ٴﵽƽ���t1ʱ��ͨ������CO2����Ӧ��Ũ����������Ӧ��������Ȼ����С���淴Ӧ����������ƽ��������Ӧ������У�����t1֮������淴Ӧ����Ϊ�����𰸣���
��4����֪ij�¶��£�Ag2SO4��M��312g/mol�����ܽ��Ϊ0.624g/100g H2O����100gˮ����Һ������ƿ�����100ml�������ʵ����ʵ�����0.624g��312g/mol��0.002mol����Ũ����0.02mol/L��������������ĵ��뷽��ʽ��֪��Һ��������Ũ�Ⱥ������Ũ�ȷֱ���0.04mol/L��0.02mol/L�����Ը��¶���Ksp��Ag2SO4����c2(Ag��)��c(SO42��)��0.042��0.02��3.2��10-5��
��5������������ʧȥ���ӣ�����������Ӧ�������õ����ӷ�����ԭ��Ӧ��������ܷ�ӦʽΪ��Fe+2H2O+2OH- ��FeO42-+3H2��֪������������Һѡ��NaOH��Һ���õ�������������Fe����ʧȥ���ӣ�ת��ΪFeO42-����������ĵ缫��ӦʽΪFe-6e+8OH- ��FeO42-+4H2O��
���㣺�����Ȼ�ѧ����ʽ��ƽ�ⳣ�������������ƽ��״̬��Ӱ�졢�ܽ�ƽ���Լ����ԭ����Ӧ��

 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�(16��)��ѧ��Ӧ�������仯�����ʡ����ǻ�ѧ�о�����Ҫ���ݡ�
��1���й��о���Ҫ�õ�C3H8(g) = 3C(ʯī,s) + 4H2(g)�Ħ�H�����ⶨʵ���ѽ��С������ͼ�ɼ���õ���
�٦�H 0����>��<��=��
�ڦ�H =
����ͼ��������Ӧ�ķ�Ӧ�ȱ�ʾ��
��2�����ᡢ�״��������������Ҫ����ԭ�ϡ����ǵ�һЩ�������£�
| ���� | HCOOH | CH3OH | HCOOCH3 |
| ��Ҫ ���� | ��ɫҺ�壬��ˮ���� K(HCOOH)>K(CH3COOH) | ��ɫҺ�壬��ˮ���� | ��ɫҺ�壬��ˮ���ܽ��С���봼���� |
 HCOOH(l) + CH3OH(l)����Ӧ���ȣ����ʱ��ֵ��С�����³�ѹ�£�ˮ�ⷴӦ���ʺ�ƽ�ⳣ������С��
HCOOH(l) + CH3OH(l)����Ӧ���ȣ����ʱ��ֵ��С�����³�ѹ�£�ˮ�ⷴӦ���ʺ�ƽ�ⳣ������С���ٹ�ҵ�����У���Ӧ��ʼ���ڼ��������ˮ�Ļ�����м�����������ͼ״����ӷ�Ӧ���ʺ��ȵĽǶȷ������Ӽ���ͼ״��Լ������ˮ���Ӱ�졣
�״��� ��
��� ��
ijС��ͨ�������о���ӦHCOOCH3ת������ʱ��仯�����ƣ����¶�T1�£�������ˮ��Ϊ1:2����ʵ�飬���ƽ����HCOOCH3��ת����Ϊ25%��
��Ԥ��HCOOCH3ת������ʱ��ı仯���Ʋ���ͼ��ʾ��

�۸÷�Ӧ���¶�T1�µ�ƽ�ⳣ��K= ��
��������λ��Ч���֣�
��3��HCOOH��Ϊ����Ĥȼ�ϵ�ص�ȼ���кܺõķ�չǰ����
д����ȼ�ϵ�صĵ缫��Ӧʽ��
��
��14�֣��й����������վ������ʾ��������(PM2.5��)Ϊ������������Ӱ�������������������Ⱦ�����ҪΪȼú��������β���ȡ���ˣ���PM2.5��SO2��NOx�Ƚ����о�������Ҫ���塣��ش��������⣺
��1����PM2.5����������ˮ�����Ƴɴ�������������ø���������ˮ���������ӵĻ�ѧ��ּ���ƽ��Ũ�����±���
| ���� | K+ | Na+ | NH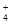 | SO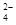 | NO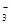 | Cl�� |
| Ũ��/mol?L��1 | 4��10��6 | 6��10��6 | 2��10��5 | 4��10��5 | 3��10��5 | 2��10��5 |
��2�� NOx������β������Ҫ��Ⱦ��֮һ����������������ʱ������N2��O2��Ӧ���������仯ʾ��ͼ���£�
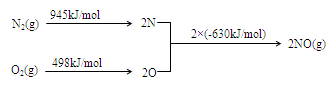
�� N2(g)��O2(g)
 2NO(g)��H�� ��
2NO(g)��H�� ���ڵ�β���п�������ʱ��NOx�ڴ�ת�����б���ԭ��N2�ų���д��NO��CO��ԭ�Ļ�ѧ����ʽ ��
�� �������Ͳ���ȫȼ��ʱ������CO���������밴���з�Ӧ��ȥCO��
2CO(g)��2C(s)��O2(g),��֪�÷�Ӧ�ġ�H��0���������ܷ�ʵ�֣� ��
��3����ѭ�����ղ���������SO2���ͻ�����Ⱦ��ͬʱ�����Ƶ������������������£�
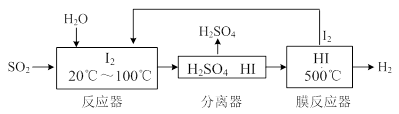
�� �����ӷ���ʽ��ʾ��Ӧ���з����ķ�Ӧ�� ����
�� �û�ѧƽ���ƶ���ԭ����������HI�ֽⷴӦ��ʹ��Ĥ��Ӧ�������H2��Ŀ���� ��
�� ������H2���ϡ������Ͻ���Ϊ��ظ�������(��MH��ʾ����NiO(OH)��Ϊ����������ϣ�KOH��Һ��Ϊ�������Һ�����Ƶø��������������������ء���س�ŵ�ʱ���ܷ�ӦΪ��Ni(OH)2��M
 NiO(OH)��MH����طŵ�ʱ�������缫��ӦʽΪ�� ���� ������ʱ��ȫ��ת��ΪNiO(OH)����������磬����һ���缫����O2��O2��ɢ����һ���缫�����缫��Ӧ�����ģ��Ӷ�������������������ر�ը��
NiO(OH)��MH����طŵ�ʱ�������缫��ӦʽΪ�� ���� ������ʱ��ȫ��ת��ΪNiO(OH)����������磬����һ���缫����O2��O2��ɢ����һ���缫�����缫��Ӧ�����ģ��Ӷ�������������������ر�ը�� (14��)��ѧ��һֱ�������о����¡���ѹ�¡��˹��̵������·���������ʵ�鱨�����ڳ��¡���ѹ�����������£�N2�ڴ���(��������Fe2O3��TiO2)������ˮ������Ӧ�����ɵ���Ҫ����ΪNH3����һ���о�NH3���������¶ȵĹ�ϵ������ʵ�����ݼ��±�(���ա�N2ѹǿ1.0��105 Pa����Ӧʱ��3 h)��
| T��K | 303 | 313 | 323 | 353 |
| NH3������/(10-6mol) | 4.8 | 5.9 | 6.0 | 2.0 |
��ش��������⣺
(1)���ڷ����ڵ�����ϵ�У�������Ӧ(I)���д�����������������·�Ӧ��������ϵ�����仯ʾ��ͼ�������б�Ҫ��ע��
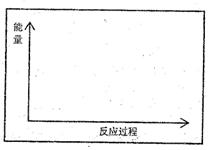
(2)��Ŀǰ�㷺ʹ�õĹ�ҵ�ϳɰ�������ȣ��÷����й̵���Ӧ�������������������䷴Ӧ����������NH3���������Ľ��飺 ��
(3)д����ҵ����H2��N2ֱ�Ӻϳ�NH3�Ļ�ѧ����ʽ ������2.0 L���ܱ������г���0.60mol N2(g)��1.60mol H2(g)����Ӧ��һ�������´ﵽƽ��ʱ��NH3�����ʵ�������(NH3�����ʵ����뷴Ӧ��ϵ�������ʵ���֮��)Ϊ4/7�������������N2��ƽ��ת����Ϊ ����Ӧ��ƽ�ⳣ��K�� (��Ҫ��д��λ)��
 2C(g) ��H��0����ͼ��ijһʱ����з�Ӧ�����뷴Ӧ���̵����߹�ϵ�������������:
2C(g) ��H��0����ͼ��ijһʱ����з�Ӧ�����뷴Ӧ���̵����߹�ϵ�������������:

 CH3OH(g)����ҵ��������CO����ȼ�ϼ״���
CH3OH(g)����ҵ��������CO����ȼ�ϼ״��� ��
�� 
 �������____________��
�������____________�� 3C(g)����֪����1molA��3molB�Ҵﵽƽ���������a molC����
3C(g)����֪����1molA��3molB�Ҵﵽƽ���������a molC����