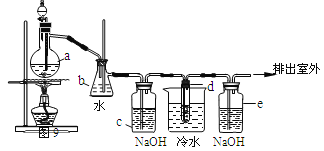
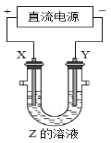
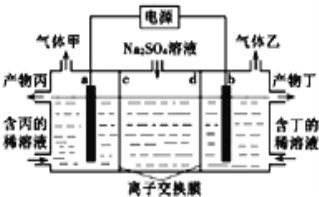
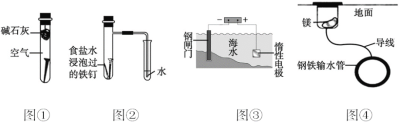
��Ŀ����
����Ŀ����֪�����£�Ksp(Ag2SO4)��2.0��10��5��ȡ����Ag2SO4��������ˮ�õ�200 mL������Һ���ڸñ�����Һ��c(SO42-)��0.017 mol��L��1����ñ�����Һ�м���0.020 mol��L��1 Na2SO4��Һ200 mL���õ���ҺR��(�����ǻ�Ϻ���Һ����ı仯)����˵����ȷ����( )
A.R��c(Ag��)��2c(SO42-)
B.�õ���ҺR�Ĺ����л��������
C.R��c(Ag��)��0.017 mol��L��1
D.����Na2SO4��Һ�ٽ������ܽ�ƽ�������ƶ�
���𰸡�C
��������
����200mL 0.020mol��L��1Na2SO4��Һ��
c��Ag����=![]() ��0.017mol��L��1��2=0.017mol��L��1��
��0.017mol��L��1��2=0.017mol��L��1��
c��SO42����=��0.017mol��L��1+0.02mol��L��1����![]() =0.0185mol��L��1��Qc=��0.017mol��L��1��2��0.0185mol��L��1=5.34��10-6��Ksp=2.0��10��5������û�г���������������Ũ�ȼ�С�����������Ũ������
=0.0185mol��L��1��Qc=��0.017mol��L��1��2��0.0185mol��L��1=5.34��10-6��Ksp=2.0��10��5������û�г���������������Ũ�ȼ�С�����������Ũ������
A��R��c(Ag��)<c(SO42-)����A����
B���õ���ҺR�Ĺ����в��������������B����
C��R��c(Ag��)��0.017 mol��L��1����C��ȷ��
D������Na2SO4��Һû�г������Dz�������Һ����D����
��ѡC��

 �ľ�ͼ���ʱ�ȷ�ϵ�д�
�ľ�ͼ���ʱ�ȷ�ϵ�д�����Ŀ��ij�о�С�����Mg(OH)2�����ܽ�����ɵ�ʵ��̽����
��2֧ʢ��1 mL 1 mol��L-1��MgCl2��Һ�и�����10��2 mol��L-1NaOH���Ƶõ���Mg(OH)2������Ȼ��ֱ������м��벻ͬ�Լ�����¼ʵ���������±���
ʵ����� | �����Լ� | ʵ������ |
�� | 4 mL 2 mol��L-1HCl ��Һ | �����ܽ� |
�� | 4 mL 2 mol��L-1NH4Cl ��Һ | �����ܽ� |
��1���ӳ����ܽ�ƽ��ĽǶȽ���ʵ���ķ�Ӧ����_____________��
��2�����ʵ���������NH4Cl��Һ�����ԣ�pHԼΪ4.5���������ӷ���ʽ�����������Ե�ԭ��___________��
��3����ͬѧ��ΪӦ����һ��ʵ�飺��ͬ����Mg(OH)2�����м�4 mL����ˮ���۲쵽�������ܽ⡣��ʵ���Ŀ����_________��
��4��ͬѧ�Dz²�ʵ����г����ܽ��ԭ�������֣�һ��NH4Cl��Һ�����ԣ���Һ�е�H+���Խ��OH- ������ʹ�����ܽ⣻����____________��
��5����ͬѧ��������ʵ�飺��4 mL 2 mol��L-1 NH4Cl��Һ�еμ�2��Ũ��ˮ���õ�pHԼΪ8�Ļ����Һ����ͬ����Mg(OH)2�����м���û����Һ���۲�����
��ʵ����֤����4���еĵڶ��ֲ²��dz����ģ���ͬѧ��õ�ʵ��������___________��
����ͬѧ�������ƻ����Һ��������___________��