��Ŀ����
����Ŀ��������Һ�У���������Ũ�ȵ�˵����ȷ���ǣ� ��
A.һ��Ũ�ȵİ�ˮ��ˮϡ�͵Ĺ����У�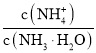 �ı�ֵ��С
�ı�ֵ��С
B.Ũ�Ⱦ�Ϊ0.1mol��L��1��Na2CO3��NaHCO3�����Һ�У�c(CO32��)<c(HCO3��)����3c(Na��)=2[c(CO32��)��c(HCO3��)��c(H2CO3)]
C.0.2mol��L��1�Ĵ���(CH3COOH)��Һ��0.1mol��L��1NaOH��Һ�������Ϻ�c(CH3COO��)��c(OH��)=c(CH3COOH)��c(H��)
D.��֪Ũ�Ⱦ�Ϊ0.1mol��L��1��NH4Cl��NH3��H2O�����Һ�Լ��ԣ���c(NH4��)��2c(H��)=c(NH3��H2O)��2c(OH��)
���𰸡�D
��������
A���������ķ�ʽ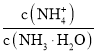 ���ӷ�ĸͬ����c(OH-)��
���ӷ�ĸͬ����c(OH-)��![]() ������Kb�dz�������ˮϡ��ʱc(OH-)��С������������ʽ��ֵ������A�����
������Kb�dz�������ˮϡ��ʱc(OH-)��С������������ʽ��ֵ������A�����
B���൱�ڽ�Na2CO3��NaHCO3�����ʵ���֮��1��1Ͷ��ˮ�У�����Һ�������غ㣨����ԭ�Ӻ�̼ԭ�Ӹ�����Ϊ3��2���ã�2c��Na+��=3��c��CO32����+c��HCO3����+c��H2CO3������������ʽ��ֻ�轫2��3��λ�û�������ȷ��B�����
C�������൱�ڽ�CH3COOH��CH3COONa�����ʵ���֮��1��1��ϣ����ݵ���غ�ã�c��Na+��+c��H+��=c��CH3COO-��+c��OH-�������������غ�ã�
c��CH3COO-��+c��CH3COOH��=2c��Na+��������1ʽ���߳���2�������2ʽ�������ȥ������Ũ�ȿɵã�c��CH3COO-��+2c��OH-��=c��CH3COOH��+2c��H+����C�����
D���൱��NH4Cl��NH3��H2O�����ʵ���֮��1��1��ϣ����ݵ���غ��c��NH4����+c��H+��=c��Cl-��+c(OH-)�����������غ�ã�c��NH4����+c��NH3��H2O��=2c��Cl-��������1ʽ���߳���2�����2ʽ�������ȥ������Ũ�ȿɵã�c(NH4��)��2c(H��)=c(NH3��H2O)��2c(OH��)��D����ȷ��
���Դ�ѡ��D�

 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�����Ŀ���ⶨ����ͭ����(CuSO4��xH2O)�ᾧˮ������ʵ���������£�
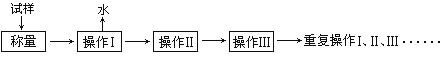
��ش�
��1��������ʵ���п����õ��ļ�������������ͼ�·�������д���������ơ�
|
|
|
|
a. ������ƽ | b. ___________ | c. ____________ | d. �ƾ���� |
��������I������������ͭ���壬���õ�����________������ţ�������
������������____________��������________������ţ��н��У��������ʵ��ⶨ���_____________������ƫ��������ƫ����������ȷ������
��2�� ���ظ�������������������Ϊ���ز������жϴﵽ���ص�������____________________________________________�����к��ز�����Ŀ����____________________________________________��
��3��ijѧ��ʵ���õ��±����ݣ�
����ǰ���� | ���Ⱥ����� | |
m1(����) | m2(����+����) | m3(����+��ˮ����ͭ) |
5.4 g | 7.9 g | 6.9 g |
�� �ɴ˸�ѧ������ó�������ͭ�����нᾧˮx��ֵΪ________________����ȷ��0.1����
�� ָ����ͬѧʵ����������ݴ����д��ڵ���Ҫ����____________������д2�㣩��