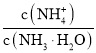��Ŀ����
����Ŀ������һ�����ʵ���Ũ�ȵ���Һ�ǻ�ѧʵ���ҵĻ���ʵ�����֮һ����ش��������⣺
��1������0.5mol/L��������Һ450mL��������Ͳ��ȡ��������98%���ܶ�1.84g/cm3��Ũ��������Ϊ___mL�����ʵ������15mL��20mL��50mL��Ͳ��Ӧ���ѡ��___mL��Ͳ��
��2������������Һ�����õ���Ͳ���ձ����������⣬����Ҫ�����ֲ���������___��
��3����������ƿ��������������������ȷŨ����Һ���������ڲ���������Һ���۲����������ȣ���ʹ��֮ǰҪ����Ƿ�©ˮ����Щ��������ȷ����__������ĸ����
A���٢ڢۢ� B���ڢ� C���٢ڢ� D���ڢۢ�
��4������ʱ������ȷ�IJ���˳����___������ĸ��ʾ��ÿ������ֻ��һ�Σ���
A��������ˮϴ���ձ�2�Ρ�3�Σ�ϴ��Һ��ע������ƿ����
B����ʢ��ˮ���ձ��м���Ũ����ϡ��
C�����ձ�������ȴ����Һ�ز�����ע������ƿ��
D��������ƿ�ǽ����������µߵ���ҡ��
E�����ý�ͷ�ιܼ�ˮ��ʹ��Һ��Һ��ǡ����̶�����
F������������ƿ��С�ļ�ˮ��ֱ��Һ��ӽ��̶�1 cm��2 cm��
��5����������������ϡ������ҺŨ��ƫ�ߵ���__������ţ���
A��Ũ����ϡ�ͺ���Һû����ȴ�����¾�ת��
B��ת��ʱû��ϴ���ձ���������
C��������ƿ��ˮ����ʱ�۾�����Һ��
D������Ͳ��Ũ�����ϴ����Ͳ����ϴ��Һת�Ƶ�����ƿ
E��ҡ�Ⱥ���Һ����ڿ̶��ߣ��ּ�����ˮ���̶���
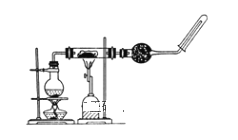
���𰸡�13.6 15 500mL����ƿ����ͷ�ι� A BCAFED ACD
��������
(1)��c=![]() ����Ũ��������ʵ���Ũ�ȣ������Һϡ���������ʵ����ʵ������������ҪŨ��������������Ũ�������ѡ����ʹ�����Ͳ��
����Ũ��������ʵ���Ũ�ȣ������Һϡ���������ʵ����ʵ������������ҪŨ��������������Ũ�������ѡ����ʹ�����Ͳ��
(2)����һ�����ʵ���Ũ�Ȳ����м��㡢����(��ȡ)���ܽ�(ϡ��)����ȴ����Һ��ϴ�ӡ����ݡ�ҡ�ȡ�װƿ�ȣ��Դ�ѡ����Ҫ��������
(3)����ƿֻ����������һ��Ũ�ȵ���Һ��ʹ��ǰ��Ҫ��©��
(4)��ϲ�����㡢����(��ȡ)���ܽ�(ϡ��)����ȴ����Һ��ϴ�ӡ����ݡ�ҡ�ȡ�װƿ������
(5)��c=![]() ��֪����������ʹnƫ���VƫС����������ҺŨ��ƫ�ߣ��Դ������
��֪����������ʹnƫ���VƫС����������ҺŨ��ƫ�ߣ��Դ������
(1)ʵ����û��450 mL������ƿ��Ӧ��ѡ��500mL������ƿ���ơ�Ũ�����Ũ��c=![]() =
=![]() mol/L=18.4mol/L��Ũ����ϡ��ǰ�����ʵ����ʵ������䣬��Ũ��������ΪV������18.4mol/L��V=0.5mol/L��0.5L��V=0.0136L=13.6mL��ѡȡ����Ͳ���Ӧ�õ��ڻ������ȡ��Һ�������ѡ15mL��Ͳ��
mol/L=18.4mol/L��Ũ����ϡ��ǰ�����ʵ����ʵ������䣬��Ũ��������ΪV������18.4mol/L��V=0.5mol/L��0.5L��V=0.0136L=13.6mL��ѡȡ����Ͳ���Ӧ�õ��ڻ������ȡ��Һ�������ѡ15mL��Ͳ��
(2)����һ�����ʵ���Ũ�ȵ���Һ�����У����㡢�������ܽ⡢��ȴת�ơ�ϴ��ת�ơ����ݡ�ҡ�ȡ�ʹ�õ��IJ��������У���Ͳ���ձ�����������500mL����ƿ����ͷ�ιܣ�
(3)����ƿ������ȷŨ����Һ�����������˳���������Һ�������������ȣ�ʹ��֮ǰҪ����Ƿ�©ˮ���ʴ�ΪA��
(4)����һ�����ʵ���Ũ�ȵ���Һ�����У����㡢�������ܽ⡢��ȴת�ơ�ϴ��ת�ơ����ݡ�ҡ�ȡ���ȷ�IJ���˳��ΪBCAFED��
(5)A��Ũ����ϡ�ͺ���Һû����ȴ�����¾�ת�ƣ��������Ƶ���Һ���ƫС��Ũ��ƫ��A��ȷ��
B��ת��ʱû��ϴ���ձ������������������ʵ����ʵ���ƫ�٣�Ũ��ƫС����B����
C��������ƿ��ˮ����ʱ�۾�����Һ�棬�������Ƶ���Һ���ƫС��Ũ��ƫ��C��ȷ��
D������Ͳ��Ũ�����ϴ����Ͳ����ϴ��Һת�Ƶ�����ƿ���������ʵ����ʵ���ƫ��Ũ��ƫ��D��ȷ��
E��ҡ�Ⱥ���Һ����ڿ̶��ߣ��ּ�����ˮ���̶��ߣ��������Ƶ���Һ���ƫ��Ũ��ƫС����E����
�ʴ�ΪACD��

����Ŀ��[��ѧһѡ��3�����ʽṹ������
(1)Ti(BH4)3��һ�ִ�����ϣ�����TiCl4��LiBH4��Ӧ�Ƶá�
�ٻ�̬Ti3+��δ�ɶԵ�������__________����
��LiBH4��Li+��BH4-������BH4-�Ŀռ乹����__________��Bԭ�ӵ��ӻ����������_____��
��ij��������ǵ������ڽ���Ԫ��M���⻯����M�IJ��ֵ��������±���ʾ:
I1/kJ��mol-1 | I2/kJ��mol-1 | I3/kJ��mol-1 | I4/kJ��mol-1 | I5/kJ��mol-1 |
738 | 1451 | 7733 | 10540 | 13630 |
M��_______(��Ԫ�ط��������ж�����Ϊ_______________��
(2)ͭ������ͭԭ�ӵĶѻ���ʽ��ͼ��ʾ��ͭ������ԭ�ӵĶѻ�ģ������____________��

(3)Aԭ�ӵļ۵����Ų�ʽΪ3s23p5��ͭ��A �γɻ�����ľ�����ͼ��ʾ���ڵ����ͭԭ������
�ٸþ���Ļ�ѧʽΪ______________��
�ڸû�����������ˮ�������ڰ�ˮ����ԭ����_____________ ���˻�����İ�ˮ��Һ��������������Ϊ����ɫ������ɫ��Һ�������ӵĻ�ѧʽΪ____________��
�ۼ�֪�þ�����ܶ�Ϊ��g��cm-3������٤������ΪNA����֪�þ�����Cuԭ�Ӻ�Aԭ��֮�����̾���Ϊ��Խ��ߵ�1/4����þ�����Cuԭ�Ӻ�Aԭ��֮�����̾���Ϊ________pm��
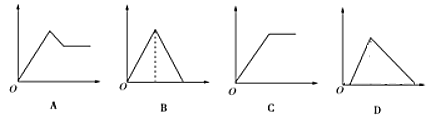
����Ŀ����ͼͼ���У�������Ϊ����������ʵ�����������Ϊ����Һ�м��뷴Ӧ������ʵ���������Ӧ��ͼ������루1������4����Ӧ����
��Һ | �������� | ��Ӧ��ͼ�� |
��1������ʯ��ˮ | ͨ����CO2���� | ____ |
��2���Ȼ�����Һ | ���������ˮ | ____ |
��3��MgCl2��AlCl3�Ļ��Һ | ��μ���NaOH��Һ������ | ____ |
��4��������NaOH��NaAlO2��Һ | ��μ���ϡ���� | ____ |
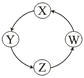
����Ŀ�����и��������У��������ͼʾ������ͨ����������һ��ת����ȫ�������
��� | X | Y | Z | W |
|
Al | NaAlO2 | Al(OH)3 | Al2O3 | ||
�� | Na | NaOH | Na2CO3 | NaCl | |
�� | C | CO | CO2 | H2CO3 | |
�� | Fe | FeCl3 | FeCl2 | Fe(OH)2 |
A. �٢� B. �٢ۢ� C. �ڢ� D. �٢�