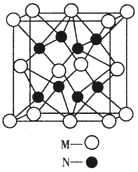
��Ŀ����
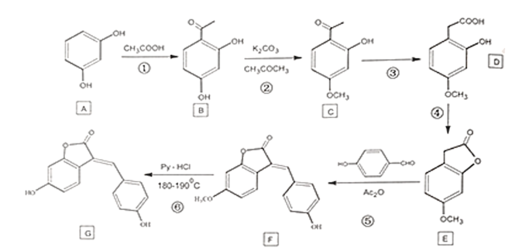
����Ŀ��������G��һ��ҩ��ϳ��м��壬��ϳ�·�ߣ�
�ش��������⣺
��1��A�Ļ�ѧ����Ϊ_________________��
��2��B�к��еĹ���������Ϊ_________________��
��3�������й�G��˵������ȷ����_________________��
A.������������ˮ
B.����ʽΪC15H9O4
C.��ʹ����KMnO4��Һ��ɫ
D.��NaOH��Ӧʱ��lmolG�������4mo1NaOH
��4��д��E��F�Ļ�ѧ��Ӧ����ʽ_________________��
��5���ķ�Ӧ����Ϊ_________________��
��6��д����������������D��ͬ���칹��Ľṹ��ʽ_________________��
������NaHCO3��Һ��Ӧ��
����ʹFeCl3��Һ����ɫ��
�ۺ˴Ź������������Ϊ1:1:2:6��
��7�������![]() ��
��![]() Ϊ��ʼԭ���Ʊ�
Ϊ��ʼԭ���Ʊ�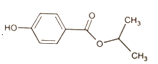 �ĺϳ�·�ߣ������Լ���ѡ����_________________
�ĺϳ�·�ߣ������Լ���ѡ����_________________
���𰸡��䱽���ӣ���1��3�������ӣ� �ǻ����ʻ� CD 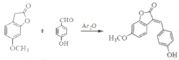 +H2O ȡ����Ӧ
+H2O ȡ����Ӧ 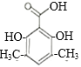 ��
��
![]()
![]()
![]()
![]()
![]()
![]()
![]()
��������
A��B���ڱ���������ȡ������COCH3��B��C�����ǻ�ת��Ϊ��OCH3��C��D����COCH3ת��Ϊ��CH2COOH��D��EΪ������Ӧ��D�����е��Ȼ����ǻ���Ӧ����������G��F����OCH3ת��Ϊ��OH��
(1)����A�ṹ��ʽ��������λ̼ԭ�����������ǻ���A�Ļ�ѧ����Ϊ�䱽����(��1��3��������)��
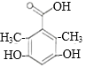
(2)����B�Ľṹ��ʽ�����еĹ���������Ϊ���ǻ����ʻ���
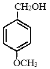
(3)A������G�Ľṹ��ʽ�����б��ӵĽṹ����ϱ��ӵ����ʣ�������һ�����ڣ�A����
B���ṹ�еĽڵ�Ϊ̼ԭ�ӣ�ÿ��̼ԭ���γ�4�����ۼ������������ԭ�Ӳ��룬�����ʽΪC15H10O4��B����
C��G�Ľṹ�к���̼̼˫��������ϩ�������ʣ���ʹ����KMnO4��Һ��ɫ��C��ȷ��
D��G�Ľṹ�к����������ǻ���һ�����������ǻ��������Կ����������Ʒ�Ӧ������������������Һ������ˮ��ʱ���γ�һ�����ǻ�������NaOH��Ӧʱ��lmolG�������4mo1NaOH��D��ȷ��
��ѡCD��
(4)����ͼʾ��E��F�Ļ�ѧ��Ӧ����ʽΪ�� +H2O��
+H2O��
(5)����ͼʾ��F�б����ϵģ�OCH3��-OHȡ���������ķ�Ӧ����Ϊȡ����Ӧ��
(6)D�Ľṹ��ʽΪ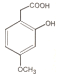 ��ͬ���칹������NaHCO3��Һ��Ӧ��˵���ṹ�к����Ȼ���ʹFeCl3��Һ����ɫ��˵���ṹ�к��б��ӵĽṹ���˴Ź������������Ϊ1:1:2:6��˵�������к���4�ֲ�ͬ��������ԭ�ӣ�����ԭ�Ӹ�����Ϊ1:1:2:6����Dͬ���칹��Ľṹ��ʽ
��ͬ���칹������NaHCO3��Һ��Ӧ��˵���ṹ�к����Ȼ���ʹFeCl3��Һ����ɫ��˵���ṹ�к��б��ӵĽṹ���˴Ź������������Ϊ1:1:2:6��˵�������к���4�ֲ�ͬ��������ԭ�ӣ�����ԭ�Ӹ�����Ϊ1:1:2:6����Dͬ���칹��Ľṹ��ʽ ��
�� ��
��
(7)�����![]() ��
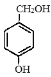
��![]() Ϊ��ʼԭ���Ʊ����������ͼ��
Ϊ��ʼԭ���Ʊ����������ͼ��![]() ��K2CO3��CH3COCH3�����·�Ӧ����
��K2CO3��CH3COCH3�����·�Ӧ����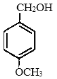 ��
��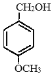 ������KMnO4��Һ����Ϊ
������KMnO4��Һ����Ϊ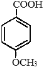 ��
��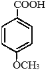 ��
��![]() ��Ũ������������·�Ӧ����
��Ũ������������·�Ӧ����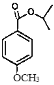 ��
��![]() �ڼ�����������Py-HCl��������
�ڼ�����������Py-HCl��������![]() ���ϳ�·�ߣ�
���ϳ�·�ߣ�
![]()

![]()
![]()
![]()
![]()
![]()
![]() ��
��