��Ŀ����
11����״���£�һ�ܱ���������Ħ�����ɻ�����������a��b�ֳɼס������ң���ͼ��ʾ���������г���0.06mol HCl�������г���H2��NH3�Ļ�����壬��ֹʱ����λ������ͼ����֪�ס������������������֮��Ϊ1.09g��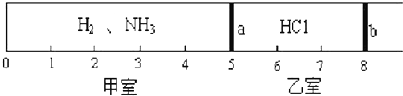
��ش��������⣺
��1����������������ʵ���Ϊ0.1mol��
��2�����������������Ϊ1.1g��
��3��������H2��NH3�����ʵ���֮��Ϊ2��3��������Ϊ4��51��
��4������������֪��HCl+NH3=NH4Cl��NH4Cl�������ǹ��壩���������aȥ������HCl��NH3��ȫ��Ӧ����b�������ڿ̶ȡ�2�����������֣���
���� ��1����ͬ�����£���������ʵ���֮�ȵ��������֮�ȣ�
��2������m=nM��������������������ٽ�ϼ��ҵ�������������������������
��3�����ݼ�������������ʵ������������㰱�������������ʵ���֮�ȡ�����֮�ȣ�
��4������ʣ����������ʵ�������ʣ��������ռ������Ӷ�ȷ��b��λ�ã�
��� �⣺��1����ͬ�����£���������ʵ���֮�ȵ��������֮�ȣ���ͼ��֪�ס�����������������Ϊ5��3���������ʵ���֮��Ϊ5��3�����Լ���������Ϊ0.1mol��
�ʴ�Ϊ��0.1��
��2��HCl���������Ϊ0.06mol��36.5g/mol=2.19g������������������Ϊ2.19-1.09g=1.1g���ʴ�Ϊ��1.1��
��3���谱�������ʵ���Ϊx�����������ʵ���Ϊy��
���������ʵ����������з�����Ϊ��$\left\{\begin{array}{l}{x+y=0.1}\\{17x+2y=1.1}\end{array}\right.$��x=0.06��y=0.04��
�����������������ʵ���֮��=0.06mol��0.04mol=3��2��
������֮��=��0.06mol��17g/mol������0.04mol��2g/mol��=51��4��
�ʴ�Ϊ��2��3��4��51��
��4��������NH3�����ʵ���Ϊ0.1mol��3/5=0.06mol��ǡ�õ���HCl�����ʵ��������Զ���ǡ����ȫ��Ӧ����NH4Cl���壬ʣ��H2�����ʵ���Ϊ0.04mol����ͬ�����£���������֮�ȵ��������ʵ���֮�ȣ����Ի���b������������2�������ʴ�Ϊ��2��
���� ���⿼���˰����ӵ����ɼ������ۣ���ȷ��ͬ�����£����������������ʵ���֮��Ĺ�ϵ�ǽⱾ��ؼ�������ϻ�����ʽ����������Ѷ��еȣ�

 �߲������Ӧ��һ��ͨϵ�д�
�߲������Ӧ��һ��ͨϵ�д�| A�� | ��ͭ�������ķ�Ӧ˳���ǣ�Fe-2e-�TFe2+��Ni-2e-�TNi2+��Cu-2e-�TCu2+�����ʽ��������Ӧ�γɡ������ࡱ | |
| B�� | ���һ��ʱ�����Һ��Cu2+��С | |
| C�� | ����·��ͨ��1 mol���ӣ�������0.5 molͭ������ | |
| D�� | ������Ӧֻ��Cu2++2e-�TCu |
| A�� | ��ʯȼ��ȼ�պ�ҵ�����еĵ���������ǵ��¡�������������Ԫ�� | |
| B�� | ����̫���ܵ������Դ���滯ʯȼ�ϣ������ڽ�Լ��Դ���������� | |
| C�� | �������������Ч��ֹȣ�� | |
| D�� | ��ѧҩƷ�Ż𣬶�Ҫ������ˮ����ĭ�������� |
| A�� | ���� | B�� | ���� | C�� | ��Һ | D�� | ��ȡ |
�ٷ�Ӧǰ����Һ������Ũ�Ȼ������ֲ������NH��Na+
���н�״��������
�����������
����Һ��ɫ�����仯
����Һ�й�������3��������ԭ��Ӧ��
| A�� | �٢ߢۢ� | B�� | �٢ۢۢܢ� | C�� | �٢ۢܢ� | D�� | �ڢܢ� |
| A�� | ��Һʱ����Һ©�����²�Һ����¶��������ϲ�Һ���©���Ͽڵ��� | |
| B�� | ��ȡ��������Ի��ܵ�Һ�����ʼ����ʷе�IJ�ͬ�����еķ��� | |
| C�� | ����ƿ������ˮϴ�Ӻ����ɺ����ʹ�� | |
| D�� | Ϊ�˱��ڲ�����Ũ��Һϡ�ͻ�����ܽ��ֱ��������ƿ�н��� |
| A�� | FeSO4��Fe�� | B�� | SnCl2��HCl�� | C�� | FeCl3��HCl�� | D�� | NaAlO2��NaOH�� |
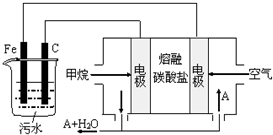 �縡ѡ���۷��ǹ�ҵ�ϲ��õ�һ����ˮ����������������ˮ��pH��5.0��6.0֮�䣬ͨ���������Fe��OH��3������Fe��OH��3�������ԣ��������� ����������������о���ˮ�����ã��������������ݰ���ˮ�����������ˮ���γɸ����㣬��ȥ����Ʋ���������㣬�����˸�ѡ���������ã�ij����С���õ縡ѡ���۷�������ˮ�����װ��ʾ��ͼ����ͼ��ʾ��
�縡ѡ���۷��ǹ�ҵ�ϲ��õ�һ����ˮ����������������ˮ��pH��5.0��6.0֮�䣬ͨ���������Fe��OH��3������Fe��OH��3�������ԣ��������� ����������������о���ˮ�����ã��������������ݰ���ˮ�����������ˮ���γɸ����㣬��ȥ����Ʋ���������㣬�����˸�ѡ���������ã�ij����С���õ縡ѡ���۷�������ˮ�����װ��ʾ��ͼ����ͼ��ʾ��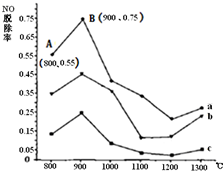 ȼú�����������еĵ�������NOx����ҪΪNO��NO2�����γ���Ⱦ�����뾭�ѳ���������ŷţ�
ȼú�����������еĵ�������NOx����ҪΪNO��NO2�����γ���Ⱦ�����뾭�ѳ���������ŷţ�