��Ŀ����
����Ŀ��һ�������£�CH4��H2O(g)������Ӧ��CH4(g)+H2O(g) ![]() CO(g)+3H2(g) ��H����ʼʱֻ����CH4��H2O(g)����
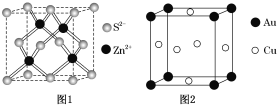
CO(g)+3H2(g) ��H����ʼʱֻ����CH4��H2O(g)����![]() =Z�����ַ�Ӧѹǿ���䣬ƽ��ʱCH4�����������(CH4)��Z��T(�¶�)�Ĺ�ϵ��ͼ��ʾ������˵����ȷ����
=Z�����ַ�Ӧѹǿ���䣬ƽ��ʱCH4�����������(CH4)��Z��T(�¶�)�Ĺ�ϵ��ͼ��ʾ������˵����ȷ����
A. a>3>b
B. �����¶Ȳ��䣬ƽ����ϵ��ѹ����(CH4)����
C. �¶�ΪT1��Z=3ʱ��Y������Ӧ�ķ�Ӧ���淴Ӧ�������
D. X��ʱ��H2���������Ϊ30%
���𰸡�BD
��������
CH4(g)+H2O(g) ![]() CO(g)+3H2(g)�������������ķ�Ӧ�������¶Ȳ��䣬ƽ����ϵ��ѹ��ƽ�������ƶ�������������������
CO(g)+3H2(g)�������������ķ�Ӧ�������¶Ȳ��䣬ƽ����ϵ��ѹ��ƽ�������ƶ�������������������
��ͼ���֪�������¶Ȳ��䣬![]() ������������������С���Դ˷�����
������������������С���Դ˷�����
A����ͼ��֪����![]() =3ʱ���Ƚϣ�����ˮ���������ʵ�����ƽ�������ƶ�����
=3ʱ���Ƚϣ�����ˮ���������ʵ�����ƽ�������ƶ�����![]() �ı�ֵԽ���������������ԽС����a<3<b����A����
�ı�ֵԽ���������������ԽС����a<3<b����A����
B.�������Ϸ������¶Ȳ���ʱ����ѹ��ƽ�������ƶ�������������������B��ȷ��
C.��ͼ��֪���¶�ΪT1��Z=3ʱ�����ڼ�����������Y�����X�㣬���Է�Ӧ������Ӧ������У���C����
D. X����Z=3��ƽ��㣬����ʼʱ����Ϊ1mol����H2O(g)Ϊ3mol��ת���ļ���Ϊamol��
CH4(g)+H2O(g) ![]() CO(g)+3H2(g)
CO(g)+3H2(g)
��ʼ��mol�� 1 3 0 0
�仯��mol�� a a a 3a
ƽ����mol��1-a 3-a a 3a
��ͼ��֪��X��ʱ��CH4���������Ϊ10% ������![]() 100%=10%�����a=0.5��
100%=10%�����a=0.5��
����H2���������=![]() 100%=
100%=![]() 100%=30%����D��ȷ��
100%=30%����D��ȷ��
��ѡBD��

 ��ĩ100�ִ��غ�������ϵ�д�
��ĩ100�ִ��غ�������ϵ�д� Сѧ�������Ծ�ϵ�д�
Сѧ�������Ծ�ϵ�д�����Ŀ����ѧ��Ӧ���������������������������
��1��Aѧ��Ϊ��̽��п�����ᷴӦ�����е����ʱ仯������200mLϡ�����м���������п�ۣ�����ˮ�������ռ���Ӧ�ų���������ʵ���¼���£��ۼ�ֵ����
ʱ�䣨min�� | 1 | 2 | 3 | 4 | 5 |
���������mL������״���� | 100 | 240 | 688 | 810 | 860 |
�ٷ�Ӧ��������ʱ����ǣ���0��1��1��2��2��3��3��4��4��5 ��_____ min��ԭ����_____________��
����2��3minʱ����������Ũ�ȱ仯����ʾ�ķ�Ӧ����Ϊ_________��������Һ������䣩
��2��Bѧ��Ҳ��ͬ����ʵ�飬�����ڷ�Ӧ̫�죬�ⲻ�������������취���ͷ�Ӧ���ʣ��������ѡ���������м�������___________�Լ�����Ӧ���ʡ�����д���ţ�
A������ B��HNO3��Һ C��CuSO4��Һ
��3��ij�¶�����10L�ܱ������У�3����̬���ʣ�A��B��C�����ʵ�����ʱ��仯������ͼ��
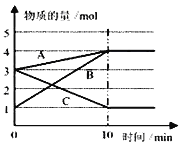
�ٸ÷�Ӧ�Ļ�ѧ����ʽ��___________
���ڸ������ﵽ��Ӧ���ȣ�ƽ��״̬��ʱ��Ӧ���ת����Ϊ____________
��ת����=��ת�������ʵ���/��ʼ���ʵ�������100%��(����������1λС����