��Ŀ����
4�������й�ʵ��˵��������ǣ�������| A�� | ��0.1mol/LK2Cr2O4��Һ�У��μ�3��Ũ���ᣬK2Cr2O4��Һ��ɫ��ı� | |
| B�� | �����¶ȶԷ�Ӧ���ʵ�Ӱ��ʵ��ʱ��Ӧ�ý�Na2S2O3��ϡ����ֱ���ȵ��趨�¶��ٻ�� | |
| C�� | ���º����Ѵ�H2��g��+I2��g��?2HI��g��ƽ��������У���������HI��g����������������ɫ���� | |
| D�� | ���϶�ȡ���ݣ�ֱ�������½���ȡ���ֵ����Ϊ�кͷ�Ӧ��Ӧ�ȵIJⶨ�л����Һ������¶� |
���� A��Cr2O72-����ɫ��+H2O?2CrO42-����ɫ��+2H+�������ᣬH+Ũ������ƽ�����ƣ�Cr2O72-Ũ������
B���¶�Ӱ�졢��Ӧ���ʵ�ʵ����ǰ��Һ���¶���ͬʱ�ٻ�ϲⶨ��Ӧ����¶ȱ仯��
C�����º����Ѵ�H2��g��+I2��g��?2HI��g��ƽ��������У���������HI��g����ƽ��������Ũ������ƽ�ⲻ�䣻
D���к��ȵIJⶨʵ���У���ȡ�����Һ������¶�Ϊ��ֹ�¶ȣ�
��� �⣺A��Cr2O72-����ɫ��+H2O?2CrO42-����ɫ��+2H+�������ᣬH+Ũ������ƽ�����ƣ�Cr2O72-Ũ��������Һ�ʳȺ�ɫ����Һ��ɫ�������Һ��ɫ�����A��ȷ��
B���¶�Ӱ�졢��Ӧ���ʵ�ʵ����ǰ��Һ���¶���ͬʱ�ٻ�ϲⶨ��Ӧ����¶ȱ仯�������¶ȶԷ�Ӧ���ʵ�Ӱ��ʵ��ʱ��Ӧ�ý�Na2S2O3��ϡ����ֱ���ȵ��趨�¶��ٻ�ϣ���B��ȷ��
C�����º����Ѵ�H2��g��+I2��g��?2HI��g��ƽ��������У���������HI��g����ƽ��������Ũ��������̬��ɫ�����C����
D���ڲⶨ�������������Ʒ�Ӧ���к���ʵ���У���ȡ�����Һ������¶ȣ���Ϊ��ֹ�¶ȣ���D��ȷ��
��ѡC��
���� ���⿼���˻�ѧƽ���Ӱ�����ط����жϣ��к��ȵIJⶨ����������ƽ���ƶ�ԭ���ǽ���ؼ�����Ŀ�ϼ�

| A�� | Cl-�Ľṹʾ��ͼ��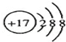 | B�� | ���������ӵĵ���ʽ��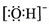 | ||
| C�� | ${\;}_{6}^{12}$C��������Ϊ12 | D�� | HClO�Ľṹʽ��H-Cl-O |
| A�� | �����������ˮ����ȡ�塢ʯ�͵ķ���ú�ĸ�������������仯 | |
| B�� | BaSO4������ˮ������������ʣ���ҽѧ���������� | |
| C�� | ���������������ڳ����½��ȶ����������еȶȵֿ�����56�治����ȫ��60��30min �����ƻ����Ⱦ�ԣ��˹�����Ҫ�����˵����ʵı��� | |
| D�� | ����������������ˮ��������ɱ�� |
| A�� | һ��������õľ�Ե�� | |
| B�� | һ���ͬ��������ǿ�ȴ� | |
| C�� | ��������ˮ���������л��ܼ� | |
| D�� | �еĸ߷��Ӳ��Ͼ����ͻ�ѧ��ʴ�����ȡ���ĥ�����͡���ˮ���ص� |
�ټ��飺��Ȼ������Ҫ�ɷ֣��ܷ���ȡ����Ӧ
����ϩ��һ������ʯ�ͻ�����չˮƽ�ı�־�����Է����ӳɷ�Ӧ
�۱���ƽ��ṹ��ÿ�������к���3��̼̼˫��
����֬�����ڸ߷��ӻ�������Է���ˮ�ⷴӦ
�ݵ��ۣ������������ʣ�����Ԫ�ر���ɫ
�����ʣ���Ũ�����Ի�ɫ��ˮ������ղ���Ϊ�����ᣮ
| A�� | �٢ڢ� | B�� | �ڢܢ� | C�� | �٢ݢ� | D�� | �٢ڢ� |
| A�� | ������ݽṹ������ֻ�ɷ�Ϊ���Ӿ��塢ԭ�Ӿ��塢���Ӿ���ͽ��������Ĵ��� | |
| B�� | �κξ����ж����л�ѧ���������Ӽ������ۼ���������������� | |
| C�� | ���������ӵľ���һ�������Ӿ��� | |
| D�� | �ɱ��ͱ������ڷ��Ӿ��� |
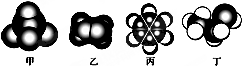
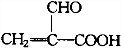
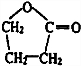
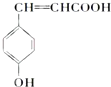
| A�� | �Ķ��ȴ���ֻ��һ�� | |
| B�� | ��������ˮ����ȡ����Ӧ��ʹ��ˮ��ɫ | |
| C�� | 1mol���к���̼̼˫������Ŀ��3NA | |
| D�� | ����ϡ���������¿�����ᷢ��ȡ����Ӧ |
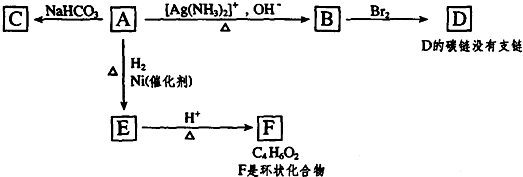
 ��
��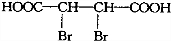 ��
�� ��
�� ��
��