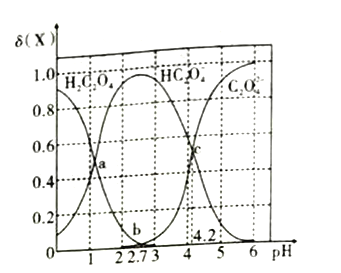
��Ŀ����
����Ŀ��ijͬѧ��NaHCO3��KHCO3��ɵĹ����������ʵ�飬���ʵ�����ݣ���������ʵ���Ũ����ȣ������ʾ������˵������ȷ���� ( )
����/mL | 50 | 50 | 50 |
������������/g | 9.2 | 15.7 | 27.6 |
��״����CO2���/L | 2.24 | 3.36 | 3.36 |
A. ��������ʵ���Ũ��Ϊ3.0 mol��L-1 B. ���ݱ��������ܼ�����������NaHCO3����������
C. ����9.2g��������ʱ������� D. 15.7g��������ǡ����������ȫ��Ӧ
���𰸡�D
��������
�������һ���������Ϊ15.7g��27.6gʱ���ɶ�����̼�����ȣ��ʻ����Ϊ27.6gʱ������ȫ��Ӧ�������15.7gʱ���ɶ�����̼���������9.2g���ɵĶ�����̼���ʻ����Ϊ9.2ʱ��������ʣ����
A.�ɱ������ݿ�֪����������Ũ��ʱ��ѡ��������������ݣ������Ź��������������ӣ�������״���¶�����̼���������������ӣ����������ѷ�Ӧ��ȫ��H����HCO===H2O��CO2����c(HCl)��![]() ��3.0 mol/L����A��ȷ��
��3.0 mol/L����A��ȷ��
B.���ݱ��е�һ�����ݹ����������������������״���µĶ�����̼�����������Լ����NaHCO3��������������B��ȷ��
C.���ڹ��������������9.2 g���ӵ�15.7 g��������״���¶�����̼��������Ҳ�����ӣ��ʼ���9.2 g��������ʱ�����������C��ȷ��
D.���ݹ�������������ı�����ϵ��֪��������״����3.36 L������̼�����������Ϊ![]() ��
��![]() �����m��13.8 g����ʱ����ǡ����ȫ��Ӧ����D������
�����m��13.8 g����ʱ����ǡ����ȫ��Ӧ����D������
��ѡD��

 �����Ծ���Ԫ���Ծ�ϵ�д�
�����Ծ���Ԫ���Ծ�ϵ�д�����Ŀ�����и������ܴ������棬��������Ӧ�Լ���ᷢ����ѧ�仯���ҷ�����Ӧ�����ӷ���ʽ��д��ȷ����( )
ѡ�� | ���飨ˮ��Һ�� | �����Լ� | ���ӷ���ʽ |
A | H+�� Na���� | Fe�� | Fe+H+=Fe3++H2�� |
B | Na����Cl���� | ������ | 2Na+2H2O=2Na��+2OH-+H2�� |
C | NH4+��H+��CH3COO- | ������ | 6H++Fe2O3=2Fe3++3H2O |
D | Ca2����OH-��Cl�� | ͨ�����CO2 | OH-+CO2= |
A. A B. B C. C D. D
����Ŀ���й�ʮ�Ŵ�ָ�����ӿ�ˮ��Ⱦ���Ρ�ʵʩ�����ͽ��������ۺ�������������Ⱦ�������ǻ�ѧ�������о�����Ҫ���⣬Ҳ�Ǽ�������ˮ��ɽ���ǽ�ɽ��ɽ������Ҫ�ٴ롣��ش��������⣺
��ѧ�о���������ǰӦ����㷺�Ļ�ʯȼ�ϵ���������Ҷ���ݽߣ������Σ������Ч;��֮һ����ʵ��ȼ�Ϻ�ȼ�ղ���֮�������ѭ��(��ͼ����ʾ)��
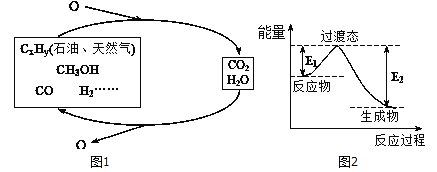
��1��һ�ֳ��õķ�������230�����д��������½�CO2��H2��ת��Ϊ�״�������ˮ������ͼ��������1molCH3OHʱ�������仯ʾ��ͼ����֪�ƻ�1mol��ͬ���ۼ�������(kJ) �ֱ����±���
C-H | C-O | C=O | H-H | H-O |
413.4 | 351 | 745 | 436 | 462.8 |
������ϱ����ݣ���д������CH3OH���Ȼ�ѧ��Ӧ����ʽ��____________________��
����֪E2=189.8kJ��mol-1����E1=_______��
��2������ͬ����CO(g) ��H2O(g) �ֱ�ͨ�뵽���Ϊ2L�ĺ����ܱ������н������·�Ӧ�� CO(g)+H2O(g)![]() CO2(g)+H2(g) �õ����±���ʾ�������ݣ�
CO2(g)+H2(g) �õ����±���ʾ�������ݣ�
ʵ�� | �¶�/�� | ��ʼ�� | �ﵽƽ�� | |||
CO/mol | H2O/mol | H2/mol | COת���� | ����ʱ��/min | ||
1 | 650 | 4 | 2 | 1.6 | 6 | |
2 | 900 | 3 | 2 |
| 3 | |
3 | 900 | |||||
���÷�Ӧ����H_______0 (����<������>��)��
��ʵ��2�����µ�ƽ�ⳣ��K=_______��
��ʵ��3�У�����ʼʱ�������м���CO��H2O��CO2��H 2��1mol�����ʱv��_______v�� (����<����>������=��)��
��3���ϳɼ״�ʱ�����CO2 ��������Na2CO3��Һ�ӹ�ҵ����(��Ҫ��CO2) �в�����ԭ����ͼ��
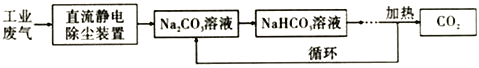
����100mL 0.1mol/L Na2CO3��Һ��ͨ��112mL (�ѻ���Ϊ��״��) CO2��������Һ��û�о����������Իش��������⣺
���ù�ҵ�����������������������װ����������______________________________��ԭ����
�������ӷ���ʽ����Na2CO3��Һ�ʼ��Ե�ԭ��_____________________��
����Ӧ��������Һ�еĸ�����Ũ���ɴ�С��˳����______________________________��