��Ŀ����
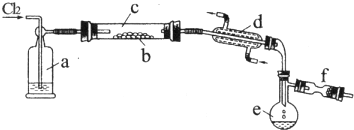
2����ͬŨ�ȵ�������п��Ӧʱ��������Ա���ԭΪSO2��Ҳ�ɱ���ԭΪ������Ϊ����֤��һ��ʵ��ijͬѧ������ͼװ�ý���ʵ�飨ʵ��ʱѹǿΪ10l kPa���¶�Ϊ0�棩��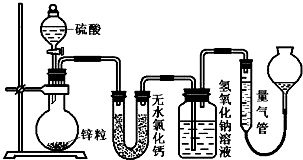
��1��������ƿ�з���1.30gп������c mol/L H2SO4��Ӧ��Ϊ��֤ʵ����۵Ŀɿ��������ܵ����˹����C��
A��200mL B��400mL C��500mL
��2����1.30gп����ȫ�ܽ⣬���ʢ����������Һ��ϴ��ƿ����l.28g����Բ����ƿ�з�����Ӧ�Ļ�ѧ����ʽΪ��Zn+2H2SO4��Ũ��$\frac{\underline{\;\;��\;\;}}{\;}$ZnSO4+SO2��+2H2O��
��3������ƿ��Ͷ��a gп����ȫ��Ӧ����������ϴ��ƿ����b g���������ռ���VmL���壬�����ݵ�ʧ�����غ��ԭ�����ɵó�a��b��V֮��Ĺ�ϵΪ����$\frac{ag}{65g/mol}$=$\frac{bg}{64g/mol}$+$\frac{��{V}_{2}-{V}_{1}��mL}{22400mL/mol}$��
��4��������ƿ��Ͷ��d gп���ټ���VL c mol/L Ũ���ᣬ��ַ�Ӧ��п��ʣ�࣬���ʢ����������Һ��ϴ��ƿ����m g��������ʵ����̲����Ļ��������H2��SO2���ʵ���֮��$\frac{n��{H}_{2}��}{n��S{O}_{2}��}$=$\frac{64cV-2m}{m}$���ú���ĸ�Ĵ���ʽ��ʾ����������ʢ����ˮ�Ȼ��Ƶ�U�ܣ�$\frac{n��{H}_{2}��}{n��S{O}_{2}��}$�ı�ֵ��ƫС����ƫ��ƫС����Ӱ�죩
���� ��1������п�������������������������Ӷ�ȷ�������ܵ����˹��
��2�������������Ƶ����ʷ������ص�ԭ��д����Ӧ�Ļ�ѧ��Ӧ����ʽ��
��3������������ԭ��Ӧ���ص������1 molп������������SO2 ���Dz�����������1 molп��Ӧ1 mol���壻
��4��������������ϴ��ƿ���ص��������Ӧ����������ʵ���������������ȥ���ɶ���������Ҫ�����������Ϊ��������������������̶����������������ʵ�����������ˮ�Ȼ��Ƶ����÷�����
��� �⣺��1��Zn+H2SO4=ZnSO4+H2��
1mol 22.4L
$\frac{1.30g}{65g/mol}$=0.02mol 0.448L
0.448L=448mL���������ܵĹ��Ӧ��ѡ500mL��
�ʴ�Ϊ��C��
��2�����������Ǽ�������������壬��������ϴ��ƿ����˵���������������ɣ���п�����ᷴӦʱ��п����ԭ����������������������ԭΪ���������ɣ�1�������֪��1.30gп�����ᷴӦ��ֻ���ɶ�����������0.02mol������Ϊ0.02mol��64g/mol=1.28g���������������ص�����һ�£��ʷ�����Ӧ�Ļ�ѧ����ʽΪ��Zn+2H2SO4��Ũ��$\frac{\underline{\;\;��\;\;}}{\;}$ZnSO4+SO2��+2H2O��
�ʴ�Ϊ��Zn+2H2SO4��Ũ��$\frac{\underline{\;\;��\;\;}}{\;}$ZnSO4+SO2��+2H2O��
��3������������ԭ֪ʶ����ݷ���ʽ��1 molп������������SO2 ���Dz�������������1molп��Ӧ1 mol���壬����������ԭ��Ӧ�е�ʧ��������ȿɵã�$\frac{ag}{65g/mol}$=$\frac{bg}{64g/mol}$+$\frac{��{V}_{2}-{V}_{1}��mL}{22400mL/mol}$��
�ʴ�Ϊ��$\frac{ag}{65g/mol}$=$\frac{bg}{64g/mol}$+$\frac{��{V}_{2}-{V}_{1}��mL}{22400mL/mol}$��
��4��Zn+2H2SO4��Ũ��=ZnSO4+SO2��+2H2O
2mol 1mol
$\frac{m}{32}$mol $\frac{m}{64}$mol
�����ɶ���������Ҫ������Ϊ$\frac{m}{32}$mol��
Zn+H2SO4=ZnSO4+H2��
1mol 1mol
�� cV-$\frac{m}{32}$��mol ��cV-$\frac{m}{32}$��mol
��$\frac{n��{H}_{2}��}{n��S{O}_{2}��}$=$\frac{��cV-\frac{m}{32}��mol}{\frac{m}{64}mol}$=$\frac{64cV-2m}{m}$��
������ʢ����ˮ�Ȼ��Ƶ�U�ܣ���������е�ˮ������������������Һ������ϴ��ƿ�������������Լ���ʱ������������ʵ������������������������ʵ���֮��ƫС��
�ʴ�Ϊ��$\frac{64cV-2m}{m}$��ƫС��
���� ���⿼����Ũ��������ʣ���Ŀ�Ѷ��еȣ���ȷ������Ӧԭ��Ϊ���ؼ���ע�����յ����غ��ڻ�ѧ�����е�Ӧ�ã�������ؿ���ѧ���ķ�����������������ѧʵ��������

 ȫ�ܲ����ĩС״Ԫϵ�д�
ȫ�ܲ����ĩС״Ԫϵ�д�| A�� | ��Na2SiO3��Һ����μ�������ϡ���SiO32-+2H+�TH2SiO3�����壩 | |
| B�� | ϡFe��NO3��2��Һ�м���ϡ���Fe2++4H++NO3-�TFe3++NO��+2H2O | |
| C�� | ��CuƬ����ϡ�����У�3Cu+8H++2NO3-�T3Cu2++2NO��+4H2O | |
| D�� | ��NH4Al��SO4��2��Һ�м��������Ba��OH��2ϡ��Һ��NH4++Al3++2SO42-+2Ba2++5OH-�T2BaSO4��+NH3•H2O+AlO2-+2H2O |
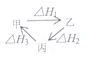
| A�� | �ס��ҡ���������ת����ͼ��ʾ�����H1=��H2+��H3 | |
| B�� | 1molCO��ȫȼ�������ȶ�������ų�����Ϊ283.0KJ����2 CO2��g��=2 CO��g��+O2��g������H=+566.0KJ•mol-1 | |
| C�� | ��N2��g��+3H2��g��?2NH3��g������H=-92.4KJ•mol-1������ij�����г���1mol N2��3molH2��ַ�Ӧ�ų�������Ϊ92.4KJ | |
| D�� | �����£�ϡHCl��ϡNaOH��Һ��Ӧ����1molH2O�ų�����Ϊ57.3 KJ�����Ȼ�ѧ����ʽΪHCl��aq��+NaOH��aq��=NaCl��aq��+H2O��aq������H=-57.3KJ |
| A�� | �٢ڢ� | B�� | �ݢޢ� | C�� | �٢ڢ� | D�� | �ۢܢ� |
| A�� | ���ú˴Ź��������Լ����Ҵ��Ͷ����� | |
| B�� |  ��һ�ȴ�����4�� ��һ�ȴ�����4�� | |
| C�� | ����飨 �������к���4�� �������к���4�� | |
| D�� | �����ϩ �� �������������9��ԭ����ͬһƽ���� �������������9��ԭ����ͬһƽ���� |
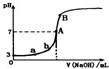
| A�� | ��ͼ��֪HA��һ�����ᣬ��Ka=1.0��10-5 | |
| B�� | ˮ�������������Ũ�ȣ�a��b | |
| C�� | ��NaOH��Һ�����Ϊ10.00mLʱ���У�c��A-��+c��OH-��=c��H+��+c��HA�� | |
| D�� | B����Һ�е�����Ũ�ȹ�ϵΪ��c��Na+����c��A-����c��OH-����c��H+�� |
 2NH3��g����H=-92��4kJ��mol-1����ʼ���ǽ�N2��H2�������20mol�������1��1������5L�ϳ����С���ӦǰѹǿΪP������Ӧ������ѹǿ��P��ʾ����Ӧ������P/P����ʱ��t�Ĺ�ϵ����ͼ��ʾ����ش��������⣺
2NH3��g����H=-92��4kJ��mol-1����ʼ���ǽ�N2��H2�������20mol�������1��1������5L�ϳ����С���ӦǰѹǿΪP������Ӧ������ѹǿ��P��ʾ����Ӧ������P/P����ʱ��t�Ĺ�ϵ����ͼ��ʾ����ش��������⣺
 ��
��