��Ŀ����
�����£�0.1mol/L��ˮ��c(H��)/c(OH��)=1��10��10������˵����ȷ����
| A����Һ����ˮ�������c(OH��)=1��10��10mol/L |
| B����Һ��c(OH��)��c��NH3��H2O��=0.1mol/L |
| C����0.1mol/L ������������Ϻ�������Һ�У�c(Cl��)=c(NH4��)��c��NH3��H2O��=0.1mol/L |
| D��ԭ��Һ�м����������Ȼ�茶�����ˮϡ�ͣ���Һ�е�c(H��)������ |
D
c(H��)c(OH��)=1��10��14��c(H��)/c(OH��)=1��10��10����c(H��)=10��12��c(OH��)=10��2��
����غ㣺c(OH��)+c��NH3��H2O��
A.��Һ����ˮ�������c(OH��)=1��10��12mol/L
B.��ˮ�����ǰ�������Һ��c(NH4+)��c��NH3��H2O��+c��NH3��=0.1mol/L
C.��0.1mol/L ������������Ϻ�������Һ���Ȼ�泥�c(Cl��)=c(NH4��)��c��NH3��H2O��=0.05mol/L.
����غ㣺c(OH��)+c��NH3��H2O��
A.��Һ����ˮ�������c(OH��)=1��10��12mol/L
B.��ˮ�����ǰ�������Һ��c(NH4+)��c��NH3��H2O��+c��NH3��=0.1mol/L
C.��0.1mol/L ������������Ϻ�������Һ���Ȼ�泥�c(Cl��)=c(NH4��)��c��NH3��H2O��=0.05mol/L.

��ϰ��ϵ�д�
�����Ŀ

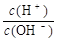 = ��
= ��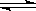 CH3COO��+ H+ ��ƽ����ϵ�У���������������ʹ����ĵ���̶Ⱥ���Һ��pH�������ǣ� ��
CH3COO��+ H+ ��ƽ����ϵ�У���������������ʹ����ĵ���̶Ⱥ���Һ��pH�������ǣ� ��