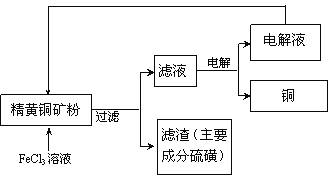
��Ŀ����
����Ŀ��Ԫ��A��B��C��D���Ƕ�����Ԫ�أ�AԪ��ԭ�ӵ�2p����Ͻ�������δ�ɶԵ��ӣ�B��3p������пչ����A��Bͬ���壬B��Cͬ���ڣ�C��ͬ�����е縺�����ģ�D����̬�⻯���ˮ��Һ��ʹ��ɫ��̪��Һ��졣�Իش�
��1��A���������ӹ���Ų�ͼΪ___��B�ĵ����Ų�ʽΪ___��C�����������Ų�ʽΪ__��D��ԭ�ӽṹʾ��ͼΪ___��
��2��D��Hԭ���γɵ�ij��̬����������ˮ��ʹ��̪��Һ��죬�����ԭ��__����֪D��Hԭ�����γ�һ�ָ������ķ���D2H2������Dԭ������8���ӽṹ��������÷��ӵĵ���ʽΪ___��
��3��B��ԭ�Ӻ�������˶�״̬��__�֣�ԭ�ӹ����Ϊ__����__��������ͬ�ĵ��ӣ�����ռ�ݵ���������ĵ��Ӳ����Ϊ__��
��4������Ԫ�����������ˮ����������ǿ�������ǣ��ö�Ӧ��ѧʽ�ش�__��
���𰸡�![]() 1s22s22p63s23p2 3s23p5
1s22s22p63s23p2 3s23p5 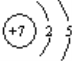 NH3+H2ONH3��H2ONH4++OH-
NH3+H2ONH3��H2ONH4++OH- ![]() 14 8 5 M HClO4>HNO3>H2CO3>H2SiO3
14 8 5 M HClO4>HNO3>H2CO3>H2SiO3
��������
��A��B��C��D���Ƕ�����Ԫ�أ�AԪ��ԭ�ӵ�2p�����ֻ���������ӣ���A����Χ�����Ų�Ϊ2s22p2����AΪCԪ�أ�B��3p������пչ������B����Χ�����Ų�Ϊ3s23p1��3s23p2��A��Bͬ���壬��BΪSiԪ�أ�B��Cͬ���ڣ�C�DZ������е縺�����ģ���CΪClԪ�أ�D����̬�⻯���ˮ��Һ��ʹ��ɫ��̪��Һ��죬��DΪNԪ�أ��Դ˽����⡣
���ݷ�����֪��AΪCԪ�أ�BΪSiԪ�أ�CΪClԪ�أ�DΪNԪ�أ�
(1)AΪCԪ�أ�ԭ������Ϊ6��������Ų�ͼΪ![]() ��
��
BΪSiԪ�أ�ԭ������Ϊ14��Siԭ�ӵĺ�������Ų�ʽΪ1s22s22p63s23p2��
CΪClԪ�أ�Clԭ�ӵĺ�������Ų�ʽΪ1s22s22p63s23p5����۵����Ų�ʽΪ3s23p5��
DΪNԪ�أ�ԭ������=�����������=7����ԭ�ӽṹʾ��ͼΪ![]() ��
��
(2)DΪNԪ�أ���HԪ����ɵ�NH3��ˮ��Һ�Լ��ԣ���NH3����ˮ���ɵ�NH3��H2O�����OH-��Ե�ʣ����뷽��ʽΪNH3+H2ONH3��H2ONH4++OH-��D2H2ΪN2H2��Nԭ������8�����ȶ��ṹ�������ʽӦΪ![]() ��
��
(3)Siԭ�Ӻ����������Ϊ14��ÿ�ֵ��ӵ��˶�״̬����ͬ�������������˶�״̬��14�֣�����Siԭ�ӵĺ�������Ų�ʽ1s22s22p63s23p2��֪����ԭ�ӹ����Ϊ8���ܼ���Ϊ5������ռ�ݵ�����ܲ����ΪM��
(4)�ǽ�����Խǿ������������Ӧˮ���������Խǿ���ǽ����ԣ�Cl��N��C��Si�������������ˮ����������ǿ������˳��Ϊ��HClO4��HNO3��H2CO3��H2SiO3��

 �������Ӧ���⼯ѵϵ�д�
�������Ӧ���⼯ѵϵ�д� �ۺ��Բ�ϵ�д�
�ۺ��Բ�ϵ�д�����Ŀ��Ԫ���ǹ������������������һ�����ʵ���ԭ��������
��1869�꣬�Ž��з���ǰ���о��Ļ������Ƴ��˵�һ��Ԫ�����ڱ�����ͼ��ʾ��
Ni=Co=59 | |||||
H=1 | Cu=63.4 | Ag=108 | Hg=200 | ||
Be=9.4 | Mg=24 | Zn=65.2 | Cd=112 | ||
B=11 | Al=27.4 | ?=68 | Ur=116 | Au=198? | |
C=12 | Si=28 | ?=70 | Sn=118 | ||
N=14 | P=31 | As=75 | Sb=122 | Bi=210? | |
O=16 | S=32 | Se=79.4 | Te=128? | ||
F=19 | Cl=35.5 | Br=80 | I=127 | ||
Li=7 | Na=23 | K=39 | Rb=85.4 | Cs=133 | Tl=204 |
Ca=40 | Pb=207 |
��1���Ž��з�����Ԫ�ذ������ԭ����������ͬһ___������������������������Ԫ���������ơ�
��2����ϱ�����Ϣ�������5�з�������Te=128�������ʺű���ĺ�����___��
��X��Y��Z��W������Ԫ�����ڱ��еĶ�����Ԫ�أ����ǵ����λ����ͼ��ʾ������YԪ��ԭ�Ӻ�������������������Ӳ�����3����
X | Y | |
Z | W |
��ش��������⣺
��1��Wλ�����ڱ��е�λ��__��W��ԭ�Ӻ�������˶�״̬��___�֣�ԭ�ӹ����Ϊ___��
��2���Ƚ�Y��Z��̬�⻯����ȶ���___���÷���ʽ��ʾ����
��3����ѧ������Ϊ���ں�������ߵĻ�����XH5��Ԥ������ˮ���ҷ�Ӧ�ų����壬����ˮ��Һ�������ԣ�д���÷�Ӧ�Ļ�ѧ����ʽ___��XH5�����ӻ�������ĵ���ʽΪ___��
��4����ҵ�Ͻ������W����ͨ�����ڵ�Z�����п��Ƶû�����Z2W2�������ʿ���ˮ��Ӧ����һ����ʹƷ����Һ��ɫ�����壬0.2mol�����ʲμӷ�Ӧʱת��0.3mol���ӣ�����ֻ��һ��Ԫ�ػ��ϼ۷����ı䣬д��Z2W2��ˮ��Ӧ�Ļ�ѧ����ʽ___��