��Ŀ����
����Ŀ����ú��Ϊȼ�Ͽ�ͨ����������;����
;��I��C(s)+O2(g)=CO2(g) ��H1<0��
;��II�����Ƴ�ˮú����C(s)+H2O(g)=CO(g)+H2(g) ��H2>0��
��ȼ��ˮú����2CO(g)+O2(g)=2CO2(g) ��H3<0��
2H2(g)+O2(g)=2H2O(g) ��H4<0��
��ش���������:
��1��;��I�ų�������_____________( ����������������������С����) ;��II�ų���������
��2����H1����H2����H3����H4����ѧ��ϵʽ��_______________��
��3��12g ̿���������в���ȫȼ������һ����̼���ų�110.35kJ���������Ȼ�ѧ����ʽΪ_______________��
���𰸡����� ��H1=��H2+![]() (��H3+��H4) C(s) +
(��H3+��H4) C(s) +![]() O2(g) = CO(g) ��H=��110.35kJ/mol
O2(g) = CO(g) ��H=��110.35kJ/mol
��������
��1���ɸ�˹���ɿ�֪������һ����Ӧ���Էֲ����У��������Ӧ�����ջ�ų��������ܺ��������Ӧһ�η���ʱ���ջ�ų���������ͬ���ʴ�Ϊ�����ڣ�
��2�����ݸ�˹���ɣ���Ӧ1=��Ӧ2+��Ӧ3��![]() +��Ӧ4��
+��Ӧ4��![]() ��������H1=��H2+
��������H1=��H2+![]() (��H3+��H4)���ʴ�Ϊ����H1=��H2+
(��H3+��H4)���ʴ�Ϊ����H1=��H2+![]() (��H3+��H4)��
(��H3+��H4)��
��3��12g ̿���������в���ȫȼ������һ����̼���ų�110.35kJ��������1mol̿���������в���ȫȼ������һ����̼���ų�110.35kJ�������Ȼ�ѧ����ʽΪ��C(s)+![]() O2(g)=CO(g)��H=-110.35 kJmol-1���ʴ�Ϊ��C(s)+
O2(g)=CO(g)��H=-110.35 kJmol-1���ʴ�Ϊ��C(s)+![]() O2(g)=CO(g)��H=-110.35kJmol-1��
O2(g)=CO(g)��H=-110.35kJmol-1��

 ��ǰ����ϵ�д�
��ǰ����ϵ�д�����Ŀ���ظ����![]() �Ǹ��л�ѧ����������������ҵ���Ը�����Ϊԭ���ü����������Ʊ�����������ͨ������
�Ǹ��л�ѧ����������������ҵ���Ը�����Ϊԭ���ü����������Ʊ�����������ͨ������![]() ��FeO��
��FeO��![]() ��
��![]() �ȣ�
�ȣ�
��֪����![]() ��ˮǿ��ˮ�⣮
��ˮǿ��ˮ�⣮
��![]() ��ɫ
��ɫ![]() ��ɫ
��ɫ![]()
��ش��������⣺
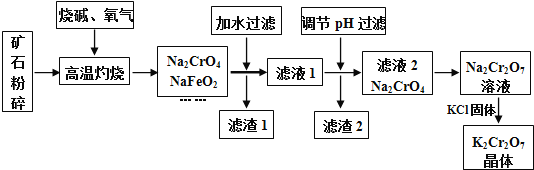
(1)����ʯ�����Ŀ���� ______ ����������ʱ![]() ������Ӧ�Ļ�ѧ����ʽΪ ______ ��
������Ӧ�Ļ�ѧ����ʽΪ ______ ��
(2)����1���к��ɫ���ʣ�д�����ɸ����ʷ�Ӧ�����ӷ���ʽ ______ ![]() ����2����Ҫ�ɷ���
����2����Ҫ�ɷ���![]() �� ______ ��
�� ______ ��
(3)�ü�Ҫ������˵��![]() ��Һ�м���KCl���壬��������
��Һ�м���KCl���壬��������![]() ��ԭ�� ______ ��
��ԭ�� ______ ��
(4)![]() ʱ���Է�Ӧ
ʱ���Է�Ӧ![]() ��ɫ
��ɫ![]() ��ɫ
��ɫ![]() ��ȡ
��ȡ![]() ��Һ����ʵ�飬��ò���ʵ���������£�
��Һ����ʵ�飬��ò���ʵ���������£�
ʱ�� | 0 |
|
|
|
|
|
|
|
|
| |
| 0 |
|
|
|
�ٷ�Ӧ�ﵽƽ��ʱ����Һ��![]() ���÷�Ӧƽ�ⳣ��KΪ ______ ��
���÷�Ӧƽ�ⳣ��KΪ ______ ��
�������й�˵����ȷ�� ______ ��
![]() ������
������![]() ���壬��ʹ��Һ�ij�ɫ����
���壬��ʹ��Һ�ij�ɫ����
![]() ʱ
ʱ![]()
![]() ��Һ��
��Һ��![]() ��
��![]() ��1ʱ�÷�Ӧ�Ѵ�ƽ��״̬
��1ʱ�÷�Ӧ�Ѵ�ƽ��״̬
![]() ��Ӧ�ﵽƽ��ʱ
��Ӧ�ﵽƽ��ʱ![]() ��ת����Ϊ
��ת����Ϊ![]()

