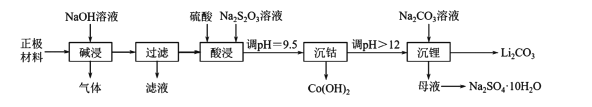
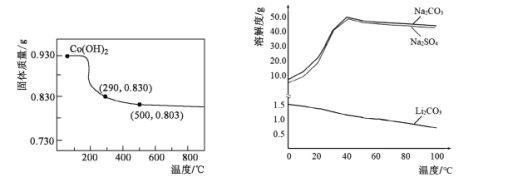
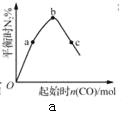
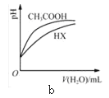
��Ŀ����
����Ŀ��ˮ�е��ܽ�����DO���Ķ����Ǻ���ˮ��ˮ�ʵ���Ҫָ�ꡣij��ѧС��ⶨij���������ĺ������������й������˽�ܽ����ⶨ����������������
������ȷ��������������ƣ�Na2S2O3����������һ�������cmol/L����Һ��
����ˮ��ƿȡ������ˮ��v1mL������������ע��1.0mLMnCl2��Һ��1.0mL����KI��Һ������ƿ����ƿ�ڲ������ݣ�����������Լ1Сʱ��
����ˮ��ƿ�м���1.0mL������Һ������ƿ������ˮ��ƿ������ȫ���ܽ⣬��ʱ��Һ��Ϊ��ɫ�� ������ˮ��ƿ����Һȫ��������ƿ�У�����������Ʊ���Һ�ζ���
V������Һ�ʵ���ɫ��1mL������Һ�������ζ����յ㲢��¼���ĵ������������Һ���Ϊv2��
��֪��I2 +2Na2S2O3 =2NaI+Na2S4O6
��1���ڵζ�������ʹ�õ������еζ��ܼС�����̨���ձ�����ƿ��________________________��
��2���ڲ�����У�ˮ���г�����MnMnO3���������ӷ���ʽΪ4Mn2++O2+8OH��![]() 2MnMnO3��+4H2O��
2MnMnO3��+4H2O��
��3��������з�����Ӧ�����ӷ���ʽΪ _______________________________________________________________��
��4���ζ�ʱ����Һ��__________ɫ��______________ɫ���Ұ��������ɫ���ٱ仯���ﵽ�ζ��յ㡣
��5����ˮ�е��ܽ���Ϊ_____________________________mg/L��
��6������ˮ�к��н϶�NO3��ʱ���ⶨ������ʵ��ֵ________(��ƫ�ߡ�ƫ�ͻ�)
���𰸡���ʽ�ζ��� MnMnO3+2I��+6H+![]() I2+2Mn2++3H2O �� ��
I2+2Mn2++3H2O �� �� ![]() ƫ��
ƫ��
��������
(1)�ζ�������ʹ�õ������еζ��ܼС�����̨���ձ�����ƿ�͵ζ��ܣ��ζ���װNa2S2O3��Һ��Na2S2O3�Լ��ԣ�
(3)������MnMnO3��������ͬʱ�е�����ʣ�࣬����1.0mL������Һ������ƿ������ˮ��ƿ������ȫ���ܽ⣬��ʱ��Һ��Ϊ��ɫ��˵������I2������������ԭ��Ӧ����ʽ������
(4)����Һ����I2���õ�����Һ��ָʾ������ҺΪ��ɫ���յ�ʱΪ��ɫ��
(5)���ݹ�ϵʽ�����ж���������
(6)���н϶�NO3��ʱ�������������£��γ����ᣬ����ǿ�����ԣ�������Na2S2O3��
(1)�ζ�������ʹ�õ������еζ��ܼС�����̨���ձ�����ƿ�͵ζ��ܣ��ζ���װNa2S2O3��Һ��Na2S2O3�Լ��ԣ��ü�ʽ�ζ��ܣ��ʴ�Ϊ����ʽ�ζ��ܣ�
(3)������MnMnO3��������ͬʱ�е�����ʣ�࣬����1.0mL������Һ������ƿ������ˮ��ƿ������ȫ���ܽ⣬��ʱ��Һ��Ϊ��ɫ��˵������I2�����⻯�ϼ����ߣ�Mn�Ļ��ϼۻή�ͣ����ӷ���ʽΪMnMnO3+2I��+6H+![]() I2+2Mn2++3H2O���ʴ�Ϊ��MnMnO3+2I��+6H+
I2+2Mn2++3H2O���ʴ�Ϊ��MnMnO3+2I��+6H+![]() I2+2Mn2++3H2O��
I2+2Mn2++3H2O��
(4)����Һ����I2���õ�����Һ��ָʾ������ҺΪ��ɫ���յ�ʱΪ��ɫ���ʴ�Ϊ�������ޣ�
(5)��4Mn2++O2+8OH��![]() 2MnMnO3��+4H2O��MnMnO3+2I��+6H+
2MnMnO3��+4H2O��MnMnO3+2I��+6H+![]() I2+2Mn2++3H2O��I2 +2Na2S2O3 =2NaI+Na2S4O6����֪��ϵʽ
I2+2Mn2++3H2O��I2 +2Na2S2O3 =2NaI+Na2S4O6����֪��ϵʽ![]() ����
����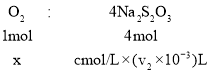 �������������ʵ���x=
�������������ʵ���x=![]() ��v1mLˮ�����ܽ���=
��v1mLˮ�����ܽ���=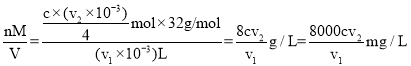 ���ʴ�Ϊ��
���ʴ�Ϊ��![]() ��
��
(6)���н϶�NO3��ʱ�������������£��γ����ᣬ����ǿ�����ԣ�������Na2S2O3����Na2S2O3�������ӣ����ƫ�ߣ��ʴ�Ϊ��ƫ�ߡ�

����Ŀ������ʵ���е���ɫ�仯����������ԭ��Ӧ�ص���
ѡ�� | A | B | C | D |
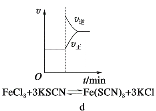
ʵ�� | NaOH��Һ����FeSO4��Һ�� | ʯ����Һ������ˮ�� | KSCN��Һ����FeCl3��Һ�� | CO2ͨ��װ��Na2O2����ĸ���� |
���� | ������ɫ����������Ϊ���ɫ | ��Һ��죬���Ѹ����ɫ | ��Һ��Ϊ��ɫ | �����ɵ���ɫ��Ϊ��ɫ |
A. A B. B C. C D. D