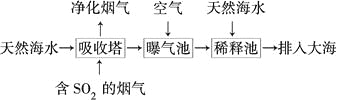
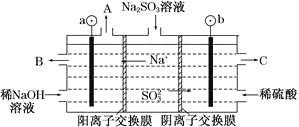
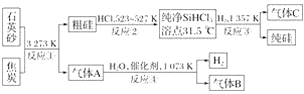
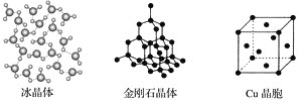
��Ŀ����
����Ŀ���ӻ�ͭ���н�ȡͭԪ�أ�����FeCl3����ȡ����
(1)��ӦCu2S+4FeCl3=2CuCl2+4FeCl2+S��ÿ����1mol CuCl2����Ӧ��ת�Ƶ��ӵ���ĿΪ_______����ȡʱ�������������¿�ά��Fe3+�ϸ�Ũ�ȡ��йط�Ӧ�����ӷ���ʽ��______________��
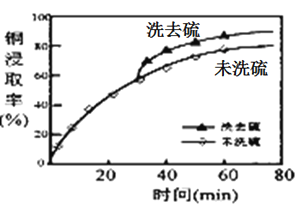
(2)��ȡ�����м���ϴ�Ӽ��ܽ���ʱ��ͭԪ�صĽ�ȡ�ʵı仯����ͼ����ԭ����_____________��
(3)353Kʱ����FeCl3��ȡҺ�м���CuCl2���ܼӿ�ͭԪ�صĽ�ȡ���ʣ��䷴Ӧԭ�����û�ѧ����ʽ��ʾΪ��________________________________________________________��CuCl+FeCl3=CuCl2+FeCl2��
(4)��ͭ����ɻ�ͭ��(��Ҫ�ɷ�ΪCuFeS2)ͨ���绯ѧ��Ӧת����ɣ��й�ת������ͼ��ת��ʱ�����ĵ缫��ӦʽΪ______________________________________________________��

���𰸡�2NA�� 1.204��1024 4Fe2++O2+4H+=4Fe3++2H2O ���ɵ�����Cu2S���棬�谭��ȡ Cu2S+2CuC12=4CuC1+S 2CuFeS2+6H++2e_=Cu2S+2Fe2++3H2S��
��������
��ͭ����S�ڸ��������գ�ʹ��ת��ΪFeS2��CuS������HCl��NaCl��CuCl2�����Һ ������ӦCu2++CuS+4Cl-=2[CuCl2]-+S�������˵õ���Һ��ͨ�����������Ӧ4CuCl2-+O2+4H+=4Cu2++8Cl-+2H2O��һ���¶��£������õ���Һ�м���ϡ���ᣬ������������ͭ���壬�ᾧ����õ�����ͭ���壬��������ԭ��Һ�õ�ͭ����������õ�FeS��S��FeS2ͨ��������յõ��������Ͷ����������������֣����������Ʊ����ᡣ
��1����ӦCu2S+4FeCl32CuCl2+4FeCl2+S����ͭ����ʧȥ��������ͭ���ӣ�������ʧȥ���������������ӵõ��������������ӣ�����2mol CuCl2����Ӧ��ת�Ƶ��ӵ���ĿΪ4mol��������1mol CuCl2����Ӧ��ת�Ƶ��ӵ���ĿΪ2mol��2NA���������������ױ�������������Ӧ��4Fe2++O2+4H+=4Fe3++2H2O���ʽ�ȡʱ�������������¿�ά��Fe3+�ϸ�Ũ�ȣ�
�ʴ�Ϊ��2NA�� 1.204��1024��4Fe2++O2+4H+=4Fe3++2H2O��
��2������ͼ��֪��δϴ��ϴȥ���ͭ�Ľ�ȡ�ʵͣ���Ϊ���ɵ�����Cu2S���棬�谭��ȡ���ʴ�Ϊ�����ɵ�����Cu2S���棬�谭��ȡ��
��3��353Kʱ����FeCl3��ȡҺ�м���CuCl2���Ȼ�ͭ������ͭ��ӦCu2S+2CuC12=4CuC1+S�����ɵ��Ȼ���ͭ���Ȼ�����ӦCuCl+FeCl3�TCuCl2+FeCl2���ܼӿ�ͭԪ�صĽ�ȡ���ʣ��ʴ�Ϊ��Cu2S+2CuC12=4CuC1+S��
��4�������õ��ӷ�����ԭ��Ӧ�����ϼ۽��ͣ�����ͼ��֪��CuFeS2�õ�������Cu2S��Fe2+��H2S���ʵ缫��ӦʽΪ��2CuFeS2+6H++2e-=Cu2S+2Fe2++3H2S�����ʴ�Ϊ��2CuFeS2+6H++2e-=Cu2S+2Fe2++3H2S����