��Ŀ����
����Ŀ�����������(Na2S2O3)���н�ǿ�Ļ�ԭ�ԣ���������ǿ�ᷴӦ���ھ�ϸ��������Ӧ�ù㷺����SO2ͨ�밴һ���������Ƴɵ�Na2S��Na2CO3�Ļ����Һ�У����Ƶ�Na2S2O3��5H2O(���մ�)��
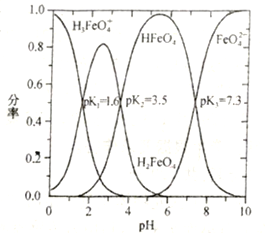
��֪��Na2S��Na2CO3��Na2SO3��NaHCO3��Һ�ʼ��ԣ�NaHSO3��Һ�����ԡ�
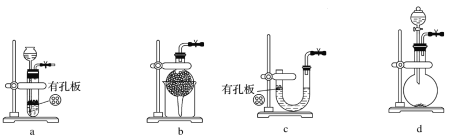
(1)ʵ������Na2SO3�������Ʊ�SO2����ѡ�õ����巢��װ����________(����ĸ����)��
(2)��Na2S��Na2CO3�Ļ����Һ�в���ͨ��SO2����Ĺ����У����֣�
��dz��ɫ�����������࣬��Ӧ�Ļ�ѧ����ʽΪ_____________________________________��
�ڵ�dz��ɫ������������ʱ����Ӧ��ϵ������ɫ��ζ�������������Ӧ�Ļ�ѧ����ʽΪ________________________________��
��dz��ɫ��������(��ʱ��Na2S2O3����)��
�ܼ���ͨ�������SO2��dz��ɫ�����ֻ������࣬��Ӧ�Ļ�ѧ����ʽΪ________________________��
(3)�Ʊ�Na2S2O3ʱ��Ϊ��ʹ��Ӧ�����������Na2S��Na2CO3�����ʵ���֮��ӦΪ____________��ͨ����Ӧ˳�ɱȽϳ����¶���ͬʱ��ͬ���ʵ���Ũ�ȵ�Na2S��Һ��Na2CO3��ҺpH�������________________��
���𰸡� d 3SO2��2Na2S===3S����2Na2SO3 SO2��Na2CO3===Na2SO3��CO2 Na2S2O3��SO2��H2O===S����2NaHSO3 2��1 Na2S��Һ
��������(1)����Na2SO3������ˮ�����װ���ж����ʵ�״̬��Ҫ������ж���
(2)�ٸ�������������Ա�������ǿ����2H2S+SO2=3S��+2H2O����������ڸ�����ɫ��ζ������ΪCO2�������������ܸ�������Na2S2O3������ǿ�ᷴӦ���������
(3)����(2)��һϵ�еķ�Ӧ����ʽ������𣻸���SO2�Ⱥ�Na2S��Ӧ������̼���Ʒ�Ӧ�����жϡ�
(1)��ΪNa2SO3������ˮ��a��b��cװ�þ�����ѡ�ã�ʵ������Na2SO3�������Ʊ�SO2����ѡ�õ����巢��װ����d���ʴ�Ϊ��d��
(2)����Na2S��Na2CO3�Ļ����Һ�в���ͨ��SO2����Ĺ����У�dz��ɫ�����������࣬�䷴Ӧԭ��ΪSO2+Na2S+H2O=H2S+Na2SO3��2H2S+SO2=3S��+2H2O������Ӧ�Ļ�ѧ����ʽΪ��3SO2+2Na2S=3S��+2Na2SO3���ʴ�Ϊ��3SO2+2Na2S=3S��+2Na2SO3��
�ڵ�dz��ɫ������������ʱ������ɫ����������������ɫ��ζ������ΪCO2���壬�仯ѧ����ʽΪSO2+Na2CO3=Na2SO3+CO2���ʴ�Ϊ��SO2+Na2CO3=Na2SO3+CO2��
�ܼ���ͨ��SO2��dz��ɫ�����ֻ������࣬Na2S2O3������ǿ�ᷴӦ������dz��ɫ�����������ԭ��Ϊ��Na2S2O3+SO2+H2O=S��+2NaHSO3���ʴ�Ϊ��Na2S2O3+SO2+H2O=S��+2NaHSO3��
(3)��3SO2+2Na2S=3S��+2Na2SO3����SO2+Na2CO3=Na2SO3+CO2����Na2SO3+S=Na2S2O3����+��+����3����4SO2+2Na2S+Na2CO3=3Na2S2O3+CO2������Na2S��Na2CO3�����ʵ���֮��Ϊ2��1����ΪSO2�Ⱥ�Na2S��Ӧ�������¶���ͬʱ��ͬ���ʵ���Ũ�ȵ�Na2S��Һ��Na2CO3��Һ��Na2S��Һ��pH���ʴ�Ϊ��2��1��Na2S��Һ��

����Ŀ��ʵ�����Ʊ�1��2����������ķ�Ӧԭ��������ʾ��
��һ����CH3CH2OH ![]() CH2=CH2��H2O��
CH2=CH2��H2O��
�ڶ�������ϩ����ˮ��Ӧ�õ�1��2���������顣
���ܴ��ڵ���Ҫ����Ӧ�У��Ҵ���Ũ����Ĵ�������140 ������ˮ��������(CH3CH2OCH2CH3)��������������������Ҵ��Ʊ�1��2�����������װ����ͼ��ʾ(����װ��δ����)��
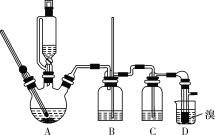
�й������б����£�
�Ҵ� | 1��2���������� | ���� | |
״̬ | ��ɫҺ�� | ��ɫҺ�� | ��ɫҺ�� |
�ܶ�/(g/cm3) | 0.79 | 2.2 | 0.71 |
�е�/(��) | 78.5 | 132 | 34.6 |
�۵�/(��) | ��130 | 9 | ��116 |
��ش��������⣺
(1)д����ϩ����ˮ��Ӧ�Ļ�ѧ����ʽ��___________________________________��
(2)�ڴ��Ʊ�ʵ���У�Ҫ������Ѹ�ٵذѷ�Ӧ�¶���ߵ�170 �����ң�������ҪĿ����___________(����ĸ����)��
a��������Ӧ��������������b���ӿ췴Ӧ����
c����ֹ�Ҵ��ӷ� d�����ٸ�������������
(3)װ��B��������__________________��
(4)��װ��C��Ӧ����_________(����ĸ����)����Ŀ�������շ�Ӧ�п������ɵ�SO2��CO2���塣
a��ˮ��������������������b��Ũ����
c������������Һ d������̼��������Һ
(5)��1��2����������ֲ�Ʒ���ڷ�Һ©���м�ˮ�����ã�����Ӧ��__________(����������������)�㡣
(6)��������������δ��Ӧ��Br2�������________(����ĸ����)ϴ�ӳ�ȥ��
a��ˮ������b������������Һ������c���⻯����Һ������d���Ҵ�
(7)�����������������������ѣ�����__________�ķ�����ȥ��
(8)�жϸ��Ʊ���Ӧ�Ѿ��������������___________________________��