��Ŀ����
����Ŀ��ij�о���ѧϰС��̽���������Һ���������������ʵ�顣
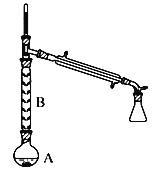
(1)ȡһ�����ı���������250 mL 0.5000 mol��L-1������Һʱ��Ҫ�õ��IJ�����������Ͳ���ձ�����������___________��___________��
(2)��ѧϰС����pH��ֽ������0.5000 mol��L-1������Һ������pH�ⶨ��������������pH��ֽ��ʹ�÷���______��
(3)������0.5000 mol��L-1�Ĵ�����Һ�ٽ���ϡ�ͣ�Ϊ�ⶨϡ�ͺ������Һ��ȷŨ�ȣ���0.2000 mol��L-1��NaOH��Һ��25.00 mL������Һ���еζ������εζ�����NaOH��Һ��������£�
ʵ����� | 1 | 2 | 3 | 4 |
����NaOH��Һ�����(mL) | 25.05 | 25.00 | 23.80 | 24.95 |
��ô�����Һ��Ũ��Ϊ________________��
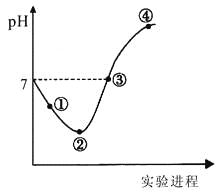
(4)ʵ��(2)�У��ζ�������pH�仯������ͼ��ʾ(����������)��

�ٵζ������У����μ�12.50mLNaOHʱ�����û����Һ������Ũ���ɴ�С˳��Ϊ____________��
�ڵ��μ�25.00mLNaOHʱ����Ӧ���û����Һ��pH=9��������Һ�У�ˮ�ĵ�����Ǵ�ˮ��_____����
(5)�ڵζ������У����в�������ɽ��ƫ�ߵ���________��
A.�ζ��յ�ʱ���ζ��ܼ��촦�а������δ��
B.��ȡNaOH��Һ���ʱ����ʼ���Ӷ������ζ��������Ӷ���
C.�ζ�����������ƿ�м�ˮ
D.�ζ���ˮϴ��δ�ñ�Һ��ϴ
���𰸡�250mL����ƿ ��ͷ�ι� ȡһС��pH��ֽ���ڱ��������Ƭ�ϣ���մ�д���Һ�IJ�������ͷ�ιܵ�����ֽ���в����۲���ɫ�ı仯���ɫ���Ա� 0.2000mol/L c(CH3COO-)>c(Na+)>c(H+)>c(OH-) 100 A D
��������
(1)��������һ�����ʵ���Ũ����Һ��ʵ�����������жϣ�
(2)����pH��ֽ��ʹ�÷������н��
(3)����c(��)V(��)=c(��)V(��)���м��㣻
(4)�����кͷ�Ӧc(��)V(��)��c(��)V(��)��ϵ���з����жϣ�
(5)����c(��)= ������������
������������
(1)����250mL0.5000mol/L������Һʱ��Ҫ�õ��IJ��������У���Ͳ���ձ�����������250mL����ƿ�ͽ�ͷ�ιܣ�
(2)pH��ֽ��ʹ�÷����ǣ�ȡһpHС����ֽ�ڱ��������Ƭ�ϣ���մ�д���Һ�IJ�������ͷ�ιܵ�����ֽ���в����۲���ɫ�ı仯���ɫ���Աȣ��ʴ𰸣�ȡһС��pH��ֽ���ڱ��������Ƭ�ϣ���մ�д���Һ�IJ�������ͷ�ιܵ�����ֽ���в����۲���ɫ�ı仯���ɫ���Աȣ�
(3)���ݱ����е����ݵ�3��ʵ���������̫������ȥ���������β�����ȥ����������Һ��ƽ�����Ϊ25.00mL������c(��)V(��)=c(��)V(��)����ô�����Һ��Ũ��c=![]() =0.2000mol/L��
=0.2000mol/L��
(4)���μ�12.50mLNaOHʱ���õ���Ũ�ȵĴ����ƺʹ���Ļ����Һ��pH<7�����ԣ�˵������ĵ���̶ȴ��ڴ�������ӵ�ˮ��̶ȣ���Һ������Ũ���ɴ�С˳��Ϊ��c(CH3COO-)>c(Na+)>c(H+)>c(OH-)��
���μ�25.00mLNaOHʱ����Ӧ��õ���������ҺpH=9����Һ��ˮ�ĵ������������������Ũ��Ϊ��![]() =10-5mol/L�������´�ˮ�ĵ���̶���10-7mol/L��������Һ��ˮ�ĵ���̶��Ǵ�ˮ�ĵ���̶�
=10-5mol/L�������´�ˮ�ĵ���̶���10-7mol/L��������Һ��ˮ�ĵ���̶��Ǵ�ˮ�ĵ���̶�![]() =100����
=100����
(5)A���ζ��յ�ʱ���ζ��ܼ��촦�а������δ���Һ�壬����NaOH��Һ���������ɲ������ƫ�ߣ���A�������⣻
B����ȡNaOH��Һ���ʱ����ʼ���Ӷ������ζ��������Ӷ�����ʹ�ö�ȡNaOH��Һ���ƫС�����´����Ũ�ȼ�С����ɽ��ƫ�ͣ���B���������⣻
C���ζ�����������ƿ�м�ˮ����Ӱ����ͼ�����ʵ��������ԶԲ��������Ӱ�죬��C���������⣻
D���ζ���ˮϴ��δ�ñ�Һ��ϴ����ɱ�ҺŨ�Ƚ��ͣ����ƫ���´����Ũ��������ɲ������ƫ�ߣ���D�������⣻
�ʴ𰸣�AD��