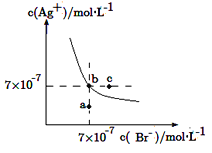
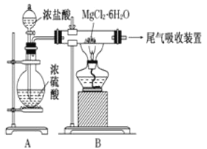
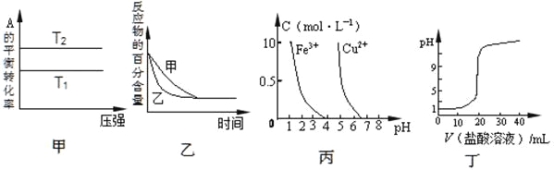
��Ŀ����
����Ŀ��(1)��CuSO4��Һ�м���һ������Na2SO3��NaCl��Һ���ȣ�����CuCl������д������CuCl�����ӷ���ʽ________________________________
(2)������H2CrO4��N2H4��ԭΪCr3����ͬʱ�ų�����Ⱦ�����壬д��������Ӧ�����ӷ���ʽ_________�����������뻹ԭ��������ʵ���֮��Ϊ________��
(3)��ȥ��Һ�е�AsCl3�����ô�������(NaH2PO2)��ԭAsCl3����������ɫ�����������H3PO3���÷�Ӧ�Ļ�ѧ��Ӧ����ʽΪ______________________�������ͻ�ԭ�������ʵ���֮��Ϊ________������������________��
(4)FeS��ˮ��Һ��Cl2���������������Һ�еμ�BaCl2�в��ܽ�������İ�ɫ�������ɣ���ˮ��Һ��FeS��Cl2��Ӧ�����ӷ���ʽΪ_______________
���𰸡�2Cu2����2Cl����SO32-��H2O![]() 2CuCl����SO42-��2H�� 4H2CrO4��3N2H4��12H��=4Cr3����3N2����16H2O 3��4 2AsCl3��3NaH2PO2��3H2O=2As����3H3PO3��3NaCl��3HCl 2��3 H3PO3 2FeS��9Cl2��8H2O=2Fe3����2SO42-��18Cl����16H��
2CuCl����SO42-��2H�� 4H2CrO4��3N2H4��12H��=4Cr3����3N2����16H2O 3��4 2AsCl3��3NaH2PO2��3H2O=2As����3H3PO3��3NaCl��3HCl 2��3 H3PO3 2FeS��9Cl2��8H2O=2Fe3����2SO42-��18Cl����16H��
��������
(1)CuSO4��Һ�м���һ������Na2SO3��Һ������ͭ���ӽ����������������Ϊ��������ӣ���������ԭΪ+1��ͭ�����ݵ�ʧ�����غ�д�����ӷ���ʽ��
(2��������H2CrO4��N2H4��ԭΪCr3+��ͬʱ�ų�����Ⱦ������Ϊ��������ϵ���غ㡢�����غ㡢ԭ���غ���ƽ��д���ӷ���ʽ;
��3����ȥ��Һ�е� AsCl3�����ô�������(NaH2PO2)��ԭAsCl3����������ɫ�����������H3PO3����ƽ��д��ѧ����ʽ����϶�����ϵ�ͻ��ϼ۱仯����÷�Ӧ���������ͻ�ԭ�������ʵ���֮�ȣ�
��4��FeS ��ˮ��Һ��Cl2���������ݵ����غ㡢ԭ���غ㡢�����غ�д�����ӷ���ʽ��
(1). CuSO4��Һ�м���һ������Na2SO3��Һ��NaCl��Һ���ȣ�����CuCl����������ͭ���ӽ����������������Ϊ��������ӣ���������ԭΪ+1��ͭ��д������ʽΪ��
2Cu2����2Cl����SO32-��H2O![]() 2CuCl����SO42-��2H��
2CuCl����SO42-��2H��
�ʴ�Ϊ��2Cu2����2Cl����SO32-��H2O![]() 2CuCl����SO42-��2H��
2CuCl����SO42-��2H��
��2��������H2CrO4��N2H4��ԭΪCr3+��ͬʱ�ų�����Ⱦ������Ϊ��������ƽ�ķ�Ӧ�����ӷ���ʽΪ��4H2CrO4��3N2H4��12H��=4Cr3����3N2����16H2O����Ӧ�и�Ԫ�ػ��ϼ۽��ͣ��õ���ԭ����Cr3+�����е�Ԫ�ػ��ϼ����ߵõ���������N2���ɷ���ʽ֪���������뻹ԭ��������ʵ���֮��Ϊ��3:4��
�ʴ�Ϊ��4H2CrO4��3N2H4��12H��=4Cr3����3N2����16H2O��3:4��
��3����ȥ��Һ�е� AsCl3�����ô�������(NaH2PO2)��ԭAsCl3����������ɫ�����������H3PO3����Ӧ�Ļ�ѧ����ʽ��3NaH2PO2+3H2O+2AsCl3��2As+3H3PO3+3NaCl+3HCl���÷�Ӧ��������AsCl3�ͻ�ԭ��NaH2PO2�����ʵ���֮��Ϊ 2:4������������H3PO3��
�ʴ�Ϊ��2AsCl3��3NaH2PO2��3H2O=2As����3H3PO3��3NaCl��3HCl ��2:3��H3PO3��
��4��FeS ��ˮ��Һ��Cl2���������������Һ�еμ�BaCl2�в��ܽ�������İ�ɫ�������ɣ�˵����Ӧ��������������ӣ���ˮ��Һ��FeS ��Cl2 ��Ӧ�����ӷ���ʽΪ��
2FeS��9Cl2��8H2O=2Fe3����2SO42-��18Cl����16H����
�ʴ�Ϊ��2FeS��9Cl2��8H2O=2Fe3����2SO42-��18Cl����16H��