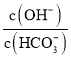��Ŀ����
����Ŀ��ʵ����������̼���ƾ���(Na2CO3��10H2O)������0.5mol�qL-1��̼������Һ1000mL���ش���������
(1)��Ҫ����̼���ƾ��������Ϊ___________g����ѡ������ƿ�Ĺ��Ϊ___________ mL��
(2)ϴ�Ӳ����У���ϴ���ձ������ҺҲע������ƿ����Ŀ����__________________________________��
(3)���ݵ���ȷ�����Ǽ���������ˮ����̶�����1~2cmʱ������_________��ˮ��Һ����̶������С�
(4)�ý�ͷ�ι�������ƿ�м�ˮʱ����С��Һ�泬���˿̶ȣ������ķ�����__________(����ĸ)��
a.��������
b.��������Һ�壬ʹ��Һ����̶�������
c.���������һ������̼���ƾ���
d.С�ļ�������ƿ����������ʹ��Һ����̶�������
(5)����ʱ���в����ᵼ��������ҺŨ��ƫ�ߵ���_____________(����ĸ)��
a.ת����ʱ��������Һ����
b.����ʱ���Ӷ�ȡ�̶�
c.����ƿ������ˮϴ����δ����
d.����ʱҺ�泬���˿̶���
(6)���������Ƶ���Һ��ȡ��10mL�����к�Na2CO3��������_____________g��
���𰸡�143.0 1000 ʹ������ȫת�Ƶ�����ƿ�� ��ͷ�ι� a b 0.53
��������
������Һ���ƵIJ�������������⣻���c=![]() ������
������
(1) ������0.5mol�qL-1��̼������Һ1000mL����Na2CO3�����ʵ���n=cV=1L��0.5molL-1=0.5mol��Na2CO310H2O�����ʵ�������Na2CO3�����ʵ���������Na2CO310H2O������0.5mol��286g/mol=143.0g����ѡ������ƿ�Ĺ��Ϊ1000mL��
(2) ����c=![]() ��֪��Ҫʹ������ҺŨ��ȷ������Ҫ����ȫ��ת�Ƶ�����ƿ�У�����ϴ�Ӳ����У���ϴ���ձ������ҺҲע������ƿ����Ŀ����ʹ������ȫת�Ƶ�����ƿ�У�
��֪��Ҫʹ������ҺŨ��ȷ������Ҫ����ȫ��ת�Ƶ�����ƿ�У�����ϴ�Ӳ����У���ϴ���ձ������ҺҲע������ƿ����Ŀ����ʹ������ȫת�Ƶ�����ƿ�У�
(3) ���ݵ���ȷ�����Ǽ���������ˮ����̶�����1-2cmʱ�����ý�ͷ�ιܼ�ˮ��Һ����̶������У�
(4) �ý�ͷ�ι�������ƿ�м�ˮʱ����С��Һ�泬���˿̶ȣ�����ʵ��ʧ�ܣ������ȣ���Ҫ��������,�ʴ�Ϊa��
(5) a��ת��ʱ��������Һ���������²���������ģ����ʵ����ʵ���ƫС����ҺŨ��ƫ�ͣ���a����
b������ʱ���Ӷ�ȡ�̶ȣ�������Һ���ƫС����ҺŨ��ƫ�ߣ���b��ȷ��
c������ƿ������ˮϴ����δ��������ʵ����ʵ�������Һ�����������Ӱ�죬��ҺŨ�Ȳ��䣬��c����
d������ʱҺ�泬���˿̶��ߣ�������Һ���ƫ����ҺŨ��ƫ�ͣ���d����
�ʴ�Ϊb��
(6) ��Һ���о�һ�ԣ�Ũ��������أ����Դ��������Ƶ���Һ��ȡ��10mL������Na2CO3�����ʵ���Ũ����0.5mol/L����̼���Ƶ�����Ϊ��0.01L��0.5mol/L��106g/mol=0.53g��