��Ŀ����
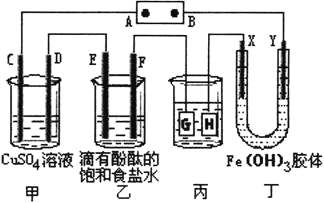
����Ŀ������ͼ��ʾ��C��D��E��F��X��Y���Ƕ��Ե缫���ס�������Һ�������Ũ�ȶ���ͬ������ͨ��ǰ����Һ������䣩��A��BΪ���ֱ����Դ����������ֱ����Դ��ͨ����X����������ɫ��dz��Y����������ɫ�����ش�
��1�����ס���װ���е�C��D��E��F�缫��ֻ��һ�ֵ�������ʱ����Ӧ���ʵ����ʵ���֮��Ϊ__________��
��2�����ñ�װ�ø�ͭ����������������Һ��pH��13ʱ����ʱ����Һ���Ϊ500mL�������жƼ���������������Ϊ__________��
��3��Fe(OH)3������Ʊ����ϸ��Ҫ��С������FeCl3��Һ�еμ�NaOH��Һ���Ʊ�Fe(OH)3���壬����ܿ�������˺��ɫ�ij������������Һ��pH=5�����ʱ��Һ��c(Fe3��)=__________mol/L������֪Ksp[Fe(OH)3]=1��10��36����
��4�����ü���ȼ�ϵ�أ��������ҺΪ2L2mol/LKOH��Һ���ṩ��Դ������ͨ����飬�ڱ�״���£����ļ�������VL��������CH4�������44.8��V��89.6ʱ����ʱ��Դ��B�������ĵ缫��ӦΪ��__________��
���𰸡���1��1��2��2��2
��2��5.4g
��3��10-9
��4��CH4��8e����9CO32����3H2O=10HCO3��
��������
�������:������������Ľ��������磬��ֱ����Դ��ͨ����X����������ɫ��dz��Y����������ɫ���˵��Y�����Դ�ĸ���������YΪ�������ɵó�D��F��H��Y��Ϊ������C��E��G��X��Ϊ������A�ǵ�Դ��������B�Ǹ�����
��1��C��D��E��F�缫�����ĵ缫��Ӧ�ֱ�Ϊ��4OH-�TO2��+2H2O+4e-��Cu2++2e-�TCu��2Cl-�TCl2��+2e-��2H++2e-�TH2���������缫ת�Ƶ��Ӿ�Ϊ1molʱ�����ɵ��ʵ����ֱ�Ϊ��0.25mol��0.5mol��0.5mol��0.5mol�����Ե��ʵ����ʵ���֮��Ϊ1��2��2��2��
��2�����װ���У��Ʋ�����������������Ƽ�������������HӦ���ǶƼ�����������Һ��pH��13ʱ����ʱ����Һ���Ϊ500mL��ʱ�����ݵ缫��Ӧ2H++2e-�TH2������ŵ�������ӵ����ʵ���Ϊ��0.1mol/l��0.5L=0.05mol����ת��0.05mol����ʱ�����жƼ���������������=108g/mol��0.05mol=5.4g��
��3����֪Ksp[Fe(OH)3]=1��10��36��pH=5����Һc(H��)=10-5mol/L��c(OH��)=1��10-14��10-5=10-9mol/L����c(Fe3��)����10-9��3=1��10��36��c(Fe3��)=10-9mol/L��
��4��n��KOH��=2mol/L��2L=4mol�������Ⱥ�����Ӧ��CH4+2O2��CO2+2H2O����CO2+2KOH=K2CO3+H2O����K2CO3+CO2+H2O=2KHCO3����44.8 L��V��89.6 Lʱ��2mol��n��CH4����4mol����2mol��n��CO2����4mol��������Ӧ�٢ڢ����õ�K2CO3��KHCO3��Һ�����ܷ�ӦʽΪ��CH4 +2O2+K2CO3�T2KHCO3+H2O�������ĵ缫��ӦʽΪCH4��8e����9CO32����3H2O=10HCO3����

 �����ߴ���ϵ�д�
�����ߴ���ϵ�д�





