��Ŀ����
4��һ�������£������Ϊ3L���ܱ������У�һ����̼��������Ӧ���ɼ״�������ΪCu2O/ZnO����CO��g��+2H2��g��?CH3OH��g����������������и��⣺
��1���������¶ȣ��״��������ʼ�С����÷�Ӧ������ӦΪ���ȷ�Ӧ�����Ȼ����ȣ���
��2���ı��������������ܼӿ컯ѧ��Ӧ���ʵ���ac��
a�����״� b������ѹǿ
c������������� d�������¶�
��3��д���������ܼӿ췴Ӧ���ʣ�����ʹһ����̼��ת������ߵĴ�ʩ������ѹǿ������������Ũ�ȣ�
���� ��1�����¼״��������ʼ�С��˵������ƽ����Щ�ƶ���
��2������Ӧ���ʵ����������¡���ѹ������Ӧ���������Ũ�ȵȵȣ��ݴ˷�����
��3�����COת����Ӧʹƽ�������ƶ���
��� �⣺��1������ƽ����Щ�ƶ���˵������Ӧ���ȣ��ʴ�Ϊ�����ȣ�
��2��a�����״�������������Ũ�ȣ���Ӧ���ʼ�������aѡ��
b������ѹǿ����Ӧ���ʼӿ죬��b��ѡ��
c�����������������Ӧ���������Ũ�Ƚ��ͣ���Ӧ���ʼ�������cѡ��
d�����·�Ӧ���ʼӿ죬��d��ѡ��
�ʴ�Ϊ��ac��
��3���÷�ӦΪ����Ӧ���������С�ķ��ȷ�Ӧ����ѹ����������Ũ���ܼӿ췴Ӧ���ʣ�����ʹһ����̼��ת������ߣ��ʴ�Ϊ������ѹǿ������������Ũ�ȣ�
���� ���⿼����Ӱ��ƽ���ƶ��ͻ�ѧ��Ӧ���ʵ����أ���Ŀ�ѶȲ���

��ϰ��ϵ�д�
�����Ŀ
14����֪±�������Ժ��Ʒ�����Ӧ�����磺2CH3CH2Br+2Na��CH3CH2CH2CH3+2NaBr��Ӧ����һ��Ӧ�����������������п������ƺϳɻ�������ǣ�������
| A�� | CH3Br | B�� | CH3CH2CH2CH2Br | ||
| C�� | CH2BrCH2Br | D�� | CH2BrCH2CH2CH2Br |
15��һ�������·�ӦA��s��+3B��g��?2C��g����10L���ܱ������н��У����2min�ڣ�A�����ʵ�����20mol��560g����С��8mol��224g����������˵������ȷ���ǣ�������
| A�� | �÷�Ӧ��A��ʾ�÷�Ӧ�ķ�Ӧ����Ϊ0.6 mol•L-1•min-1 | |
| B�� | �÷�Ӧ��B��ʾ�÷�Ӧ�ķ�Ӧ����Ϊ2.4 mol•L-1•min-1 | |
| C�� | 2v��B��=3v��C�� | |
| D�� | ��������C��ʾ�÷�Ӧ�ķ�Ӧ����Ϊ3.6 mol•L-1•min-1 |
19�������йص������Һ���������ʵ���Ũ�ȹ�ϵ��ȷ���ǣ�������
| A�� | ��0.1mol/LNaHCO3��Һ�У�c��Na+����c��HCO3-����C��CO32-����c��H2CO3�� | |
| B�� | ��0.1mol/LNa2CO3��Һ�У�c��OH-��-c��H+��=c��HC03-��+c��H2CO3�� | |
| C�� | ��0.2mol/LNaHCO3��Һ�м�������0.1mol/LNaOH��Һ��c��CO32-����c��HCO3-����c��OH-����c��H+�� | |
| D�� | �����£�CH3COONa��CH3COOH�����Һ[pH=7��c��Na+��=0.1mol/L]��c��Na+��=c��CH3COO-����c��CH3COOH����c��H+��=c��OH-�� |
9��ijʵ��С���H2O2�ķֽ���������̽�����±��Ǹ�ʵ��С���о�Ӱ��H2O2�ֽ����ʵ�����ʱ��¼��һ�����ݣ���������ͬ��״̬��ͬ��MnO2�ֱ����ʢ��15ml 5%��H2O2��Һ�Ĵ��Թ��У����ô����ǵ�ľ�����ԣ�������£�
��1��д�����Թ��з�����Ӧ�Ļ�ѧ����ʽ��2H2O2 $\frac{\underline{\;MnO_{2}\;}}{\;}$2H2O+O2�����÷�Ӧ�Ƿ��ȷ�Ӧ������Ȼ����ȣ���
��2��ʵ���������������Ĵ�Ч��������Ӵ���� �йأ�
| MnO2 | �����Թ���� | �۲��� | ��Ӧ��������ʱ�� |
| ��ĩ״ | ���� | ���ҷ�Ӧ�������ǵ�ľ����ȼ | 3.5min |
| ��״ | �� | ��Ӧ���������Ǻ�����ľ��δ��ȼ | 30min |
��2��ʵ���������������Ĵ�Ч��������Ӵ���� �йأ�

 ��֪AΪ�������ʣ��������б仯����������⣺
��֪AΪ�������ʣ��������б仯����������⣺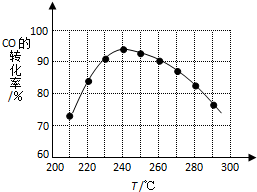 �����ѣ�CH3OCH3����δ������������ͺ�Һ������Ϊ�ྻҺ��ȼ��ʹ�ã���ҵ����CO��H2Ϊԭ������CH3OCH3���¹�����Ҫ����������Ӧ��
�����ѣ�CH3OCH3����δ������������ͺ�Һ������Ϊ�ྻҺ��ȼ��ʹ�ã���ҵ����CO��H2Ϊԭ������CH3OCH3���¹�����Ҫ����������Ӧ��