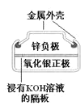
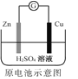
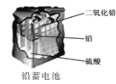
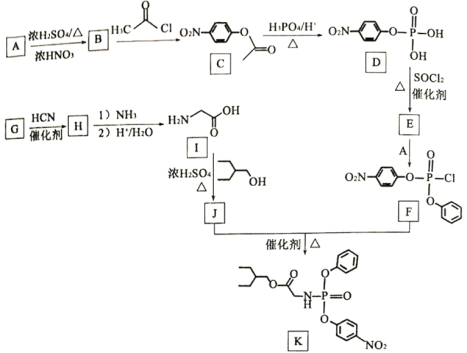
��Ŀ����
����Ŀ��������ͭ[Cux(Met)y��Met��ʾ�����������]��һ�������������Ӽ���Ϊȷ��������ͭ[Cux(Met)y]����ɣ���������ʵ�飺
(1)��ȡһ����������Ʒ����ƿ�У���������������ˮ��ϡ���ᣬ������ȫ���ܽ⣬��ȴ����Һ�ֳ����ȷݡ�
��ȡ����һ����Һ��������ҺpH��6��8֮�䡣����0.1000 mol/LI2�ı���Һ25.00 mL,��ַ�Ӧ�����2��3��ָʾ��X����0.1000 mol/LNa2S2O3����Һ�ζ�����ɫǡ����ȥ��������Ӧ��![]() ������Na2S2O3����Һ22.00 mL(��������I2��Ӧʱ���ʵ���֮��Ϊ1��1�����ﲻ��Na2S2O3������Ӧ)��
������Na2S2O3����Һ22.00 mL(��������I2��Ӧʱ���ʵ���֮��Ϊ1��1�����ﲻ��Na2S2O3������Ӧ)��
������һ����Һ�м���NH3��H2O-NH4Cl������Һ��������70�����ң�����2-3��ָʾ��PAN����0.02500 mol/LEDTA (Na2H2Y)����Һ�ζ�����Cu2+(���ӷ���ʽΪCu2++H2Y2--=CuY2-+2H+)������EDTA����Һ28.00 mL��
(1)ָʾ��XΪ ____��
(2)��Na2S2O3��Һ�ζ�ʱ����pH��С������S��SO2���ɡ�д��S2O32-��H+��Ӧ�����ӷ���ʽ ___________ ��
(3)���ζ���ˮϴ��δ��EDTA����Һ��ϴ�����Cu2+�����ʵ�����____(�ƫ����ƫС�����䡱)��
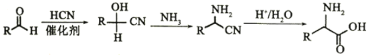
(4)ͨ������ȷ��������ͭ[Cux(Met)y]�Ļ�ѧʽ(д���������)________��
���𰸡�������Һ ![]() ƫ��
ƫ�� ![]()
![]()
![]()
![]()
![]()
![]()
![]() ��ѧʽΪ��
��ѧʽΪ��![]()
��������
��1����0.1000 mol/LNa2S2O3����Һ�ζ�I2Һ����ָʾ��Ϊ������Һ��
�ʴ�Ϊ��������Һ��
��2��S2O32-��H+��Ӧ����S��SO2����ѧ����ʽΪ![]() ��
��
�ʴ�Ϊ��![]() ��
��
��3�����ζ���ˮϴ��δ��EDTA����Һ��ϴ����Һ���ƫ���Cu2+�����ʵ�����ƫ��
�ʴ�Ϊ��ƫ��
��4��![]()
![]()
![]()
![]()
![]()
![]()
![]() ��ѧʽΪ��
��ѧʽΪ��![]()

 ���ɿ��õ�Ԫ������ĩר����100��ϵ�д�
���ɿ��õ�Ԫ������ĩר����100��ϵ�д�