��Ŀ����
����Ŀ��������Һ��Ũ�ȹ�ϵ��ȷ����(����)
A. С�մ���Һ�У�c(Na��)��c(H��)��c(HCO)��c(CO![]() )��c(OH��)
)��c(OH��)
B. CH3COONa��Һ�У�c(CH3COO��)>c(Na��)
C. ���ʵ���Ũ����ȵ�CH3COOH��Һ��CH3COONa��Һ�������ϣ�c(CH3COO��)��2c(OH��)��2c(H��)��c(CH3COOH)
D. 0.1 mol/L��NaHA��Һ����pH��4����c(HA��)>c(H��)>c(H2A)>c(A2��)
���𰸡�C
��������A.��ɲ��غ㣬����B. CH3COONa��Һ�У����������Ҫˮ�⣬c(CH3COO��)��c(Na��)������C. ���ʵ���Ũ����ȵ�CH3COOH��Һ��CH3COONa��Һ�������ϣ��������غ㣺2c(Na��)��c(CH3COO��)��c(CH3COOH)�͵���غ���c(Na��)��c(H��)��c c(CH3COO��)��c(OH��)����ʽ���е���c(CH3COO��)��2c(OH��)��2c(H��)��c(CH3COOH)��C��ȷ��D. 0.1 mol/L��NaHA��Һ����pH��4��˵��HA���ĵ������ˮ�⣬��c(HA��)>c(H��)> c(A2��)> c(H2A)��D������

 �Ķ��쳵ϵ�д�
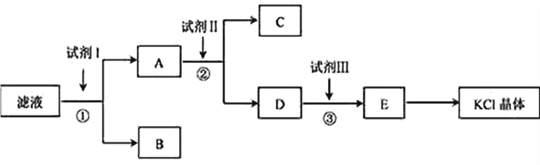
�Ķ��쳵ϵ�д�����Ŀ��ij��ɫϡ��ҺX�У����ܺ����±����������е�ij���֡�
������ | CO |
������ | Al3����Fe3����Mg2����NH |
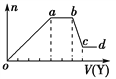
��ȡ����Һ�����������м���ij�Լ�Y���������������ʵ���(n)������Լ����(V)�Ĺ�ϵ��ͼ��ʾ��
��1����Y�����ᣬ��oa��ת��Ϊ����������(ָ��Դ��X��Һ�ģ���ͬ)��________�� bc�η�����Ӧ�����ӷ���ʽ��________________________��
��2����Y��NaOH��Һ����X��һ�����е�������______________________��bc�η�����Ӧ�����ӷ���ʽ��_____________________________________��