��Ŀ����
10��ij��ѧ����С�����Ⱦ�����IJ��ַǽ������������̽�����������ĿҪ��ش��������⣮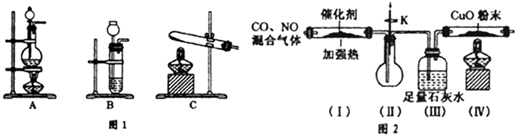
��1�������ϵ�֪��HCOOH$��_{��}^{Ũ�����}$ CO+H2O��ʵ��������ͼl��ʾ��װ�ã���ȡCO�����ѡ�õ�װ��ΪA������ţ�
��2�������ϵ�֪�����ô�����ʹ����β���е�һ����̼�͵�������ַ�����Ӧת��Ϊ������̼�͵�������С����ʵ����ģ������β���������������ͼ2��ʾװ�ã����ּгֺ�װ������ȥ����
��ʵ��ǰ���ر�����K����ͨ�����ž�װ���еĿ�������Ŀ���Ƿ�ֹCO�������ϼ��ȱ�ը��
��װ�ã�III������Ҫ�����Ǽ���CO�Ƿ�ת��ΪCO2��
�۸���װ�����в�����֮������Ӧ��װ�ã�������β������װ�ã���������β���к��еĻ�����CO�ķ����ǣ�������PdC12��Һ��ͨ������β���������ɺ�ɫ������Pd����֤������β���к���CO��д����Ӧ�����ӷ���ʽCO+Pd2++H2O=Pd+CO2+2H+��
���� ��1��ѡ������ķ���װ�ø��ݷ�Ӧ���״̬�ͷ�Ӧ������
��2��ģ������β���������̣�װ�ã�I����CO��NO�������ͨ������������Ӧ��2CO+2NO$\frac{\underline{����}}{��}$2CO2+N2��ʵ��ǰ���ر�����K����ͨ�����ž�װ���еĿ�������ֹ��ȼ�������������������Ϸ�����ը��װ�ã�II���������������٣�װ�ã�III��������CO�Ƿ�ת��ΪCO2��װ�ã�������һ����̼������ͭ��Ӧ��һ����̼��һ�������ж������Է�ֹһ����̼��һ������й©�Ӷ���Ⱦ��������Ӧ��װ�ã�������β������װ�ã�
�ٿ�ȼ�������ڵ�ȼǰ����Ҫ�鴿����ֹ������ը��
��CO2�������ʯ��ˮ����ǣ�
��������PdC12��Һ��ͨ������β���������ɺ�ɫ������Pd����PdC12����ԭ��Pd��CO��������ΪCO2���ݴ���д���ӷ���ʽ��
��� �⣺��1�����ݷ�Ӧ����ʽHCOOH$��_{��}^{Ũ�����}$ CO+H2O��֪���÷�ӦΪҺ�塢����װ�ã�Ӧ��ѡ��װ��A����Һ©����ʢ�ż��ᣬͨ����Һ©���������Ƶμӵ����ʿ��Ʒ�Ӧ�����ʣ�
�ʴ�Ϊ��A��
��2����ʵ��ǰ���ر�����K����ͨ�����ž�װ���еĿ�������ֹ��ȼ�������������������Ϸ�����ը��
�ʴ�Ϊ����ֹCO�������ϼ��ȱ�ը��
��CO2�������ʯ��ˮ����ǣ���һ����̼���ܣ�����װ�ã�III������Ҫ�����Ǽ���CO�Ƿ�ת��ΪCO2��
�ʴ�Ϊ������CO�Ƿ�ת��ΪCO2��
��3������β���к���CO��������PdCl2��Һ��ͨA����β�������ɺ�ɫ������Pd��������������ԭ��Ӧ��֪CO��������ΪCO2������Ԫ���غ��֪��ˮ�μӡ�������HCl����Ӧ�����ӷ���ʽΪ��CO+Pd2++H2O=Pd+CO2+2H+��
�ʴ�Ϊ��CO+Pd2++H2O=Pd+CO2+2H+��
���� ���⿼��������ʵ�鷽������������ۣ���Ŀ�Ѷ��еȣ���ȷʵ��ԭ������ѧʵ�������������Ϊ���ؼ��������ֿ�����ѧ���ķ�����������������ѧʵ��������ע��������������ʵ�鷽�����������ԭ��

| A�� | ������ͬŨ�ȵ�����HX��HY��ǰ�ߵ�Ka��С����Һ����ˮϡ����ͬ����ʱ��HY��Һ��pH�ı�ֵС��HX��Һ��pH�ı�ֵ | |
| B�� | ��NH3•H2O��NH4ClŨ�Ⱦ�Ϊ0.1 mol•L-1����ϵ�У������������ʱ����Һ��pH�ɱ��ֻ������� | |
| C�� | ��NaH2PO4ˮ��Һ�д��ڹ�ϵ��c��H3PO4��+c��H+��=c��HPO42-��+c��PO43-��+c��OH-�� | |
| D�� | �����ܽ�ƽ��AgI?Ag++I- ��ƽ�ⳣ��Ϊ8.5��10-17��˵��������AgI��������� |
| A�� | 4 mol | B�� | 3.4 mol | C�� | 2.8 mol | D�� | 1.2 mol |
| A�� | ������ˮ��Ӧ��Cl2+H2O�TCl-+2H++ClO- | |
| B�� | ����ͨ�������Һ�У�NH3+H+�TNH4+ | |
| C�� | �Ʊ�Fe��OH��3���壺Fe3++3H2O $\frac{\underline{\;\;��\;\;}}{\;}$ Fe��OH��3�����壩+3H+ | |
| D�� | ̼��������Һ�м������ʯ��ˮ��HCO3-+OH-�TCO32-+H2O |
| A�� | 0��lmol���麬�еĵ�����ΪNA | |
| B�� | lL 0.1mol/L Na2 CO3��Һ�к��е�CO32-��ĿΪ0.1NA | |
| C�� | 1L pH=l��������Һ�к��е�H+��Ϊ0.2NA | |
| D�� | ��״���£�2.24L CO��CO2��������к��е���ԭ����Ϊ0.15NA |
��һ����ͬѧ���������ʵ����ò���ҩƷ���Ƿ���Fe2+��̽��Vc�����ã�
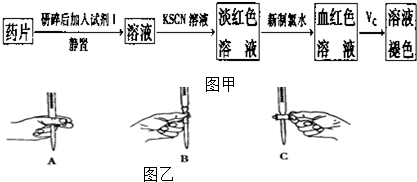
��������ͬѧ�����������������ø�����ر���Һ�ζ��ķ����ⶨ��ҩƷ�Ƿ�ϸ�Ӧԭ��Ϊ5Fe2++8H++MnO-4�T5Fe3++Mn2++4H2O��ȷ��������ҩƷ10.00g������ȫ�������Լ�2�У����Ƴ�1000mL��Һ��ȡ��20.00mL���� 0.020mol/L��KMnO4��Һ�ζ�����ȥKMnO4��Һ��¼���£�
| ����Һ �����mL�� | ��Һ | |||||
| Ũ�ȣ�mol/L�� | �ζ�ǰ | �ζ��� | �����mL�� | ƽ��ֵ��mL�� | ||
| ��һ�� | 20.00 | 0.0200 | 0.00 | 14.30 | ||
| �ڶ��� | 0.20 | 12.22 | ||||
| ������ | 0.00 | 11.98 | ||||
��2����ҩƷ���������ĺ�����33.6%������ֵ�����������̣�
��3����ʵ���е��Լ�2���ͬѧ��Ƶ�ʵ���е��Լ�1��������C�����ţ�
A������ˮ B��ϡ���� C��ϡ���� D��ϡ����
��4����ʵ��ζ������еζ������ø�����ر���Һ��ϴ�����������������ʽ�ζ����м���1-2mLKMnO4��Һ�����ζ��ܺ������ת������KMnO4��Һ�����ζ����ڱڣ�������ŵ���ϴҺ���ظ�����2-3�Σ�
�����ζ��ܵ�ͼʾ��ȷ����A�����ţ�
��5��ijͬѧ���ñ�NaOH��Һ�ζ�HCl��Һ�ⶨ��Ũ�ȣ����в��������ᵼ��ʵ����ƫ�ߵ���df
a����ʽ�ζ�����װ��Һǰδ�ô���������Һ��ϴ��
b����ʼʵ��ʱ��ʽ�ζ��ܼ��첿��û�����ݣ��ζ�������������
c����ƿ����Һ��ɫ�仯����ɫ��Ϊ�ۺ�ɫ���������µζ���Һ�����ڿ̶ȣ�
d��ʢ������Һ����ƿ�ζ�ǰ�ô���ҺҺ��ϴ��
e����ƿ��װ������ּ�����������ˮ��ζ���
f�����ú�����NaCl��NaOH�������Ʊ���Һ�����ζ��������ᣮ
| A�� | ���������ʵ���Ũ����ȵĢ٣�NH4��2CO3��Һ���ڣ�NH4��2SO4��Һ�͢�NH4Cl��Һ�У�ˮ�ĵ���̶ȣ��٣��ۣ��� | |
| B�� | �����½������ơ���������Һ��Ϻ���Һ�����ԣ�������Һ�У�c��Na+��=c��Cl-�� | |
| C�� | ij��Һ��ˮ�������c��H+��=1.0��10-12mol/L�������Һ��K+��HCO3-��Cl-��SO32-���Դ������� | |
| D�� | 0.1mol��������������������Һ�����£���Ӧ��ת�Ƶ���0.1NA |
 ���������г��ù��������������ⶨ�����̵ĺ�������Ӧԭ��Ϊ��2Mn2++5S2O82-+8H2O$\frac{\underline{\;Ag+\;}}{\;}$2MnO4-+10SO42-+16H+
���������г��ù��������������ⶨ�����̵ĺ�������Ӧԭ��Ϊ��2Mn2++5S2O82-+8H2O$\frac{\underline{\;Ag+\;}}{\;}$2MnO4-+10SO42-+16H+