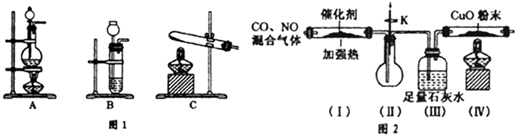
��Ŀ����
19��������Ѫ�쵰����Ҫ��ɳɷ֣�������������֯����O2�����ã����ȱ���Ϳ��ܳ���ȱ����ƶѪ���������˹�������Ҳ�к���������һ�ֳ�������ҩƷ˵�����еIJ������ݣ���ҩƷ��Fe2+33%��36%��������ˮ�������������е�θ���Vc��ά����C��ͬ�������ӱ�Ʒ���գ���һ����ͬѧ���������ʵ����ò���ҩƷ���Ƿ���Fe2+��̽��Vc�����ã�
��������ͬѧ�����������������ø�����ر���Һ�ζ��ķ����ⶨ��ҩƷ�Ƿ�ϸ�Ӧԭ��Ϊ5Fe2++8H++MnO-4�T5Fe3++Mn2++4H2O��ȷ��������ҩƷ10.00g������ȫ�������Լ�2�У����Ƴ�1000mL��Һ��ȡ��20.00mL���� 0.020mol/L��KMnO4��Һ�ζ�����ȥKMnO4��Һ��¼���£�
| ����Һ �����mL�� | ��Һ | |||||
| Ũ�ȣ�mol/L�� | �ζ�ǰ | �ζ��� | �����mL�� | ƽ��ֵ��mL�� | ||
| ��һ�� | 20.00 | 0.0200 | 0.00 | 14.30 | ||
| �ڶ��� | 0.20 | 12.22 | ||||
| ������ | 0.00 | 11.98 | ||||
��2����ҩƷ���������ĺ�����33.6%������ֵ�����������̣�
��3����ʵ���е��Լ�2���ͬѧ��Ƶ�ʵ���е��Լ�1��������C�����ţ�
A������ˮ B��ϡ���� C��ϡ���� D��ϡ����
��4����ʵ��ζ������еζ������ø�����ر���Һ��ϴ�����������������ʽ�ζ����м���1-2mLKMnO4��Һ�����ζ��ܺ������ת������KMnO4��Һ�����ζ����ڱڣ�������ŵ���ϴҺ���ظ�����2-3�Σ�
�����ζ��ܵ�ͼʾ��ȷ����A�����ţ�
��5��ijͬѧ���ñ�NaOH��Һ�ζ�HCl��Һ�ⶨ��Ũ�ȣ����в��������ᵼ��ʵ����ƫ�ߵ���df
a����ʽ�ζ�����װ��Һǰδ�ô���������Һ��ϴ��
b����ʼʵ��ʱ��ʽ�ζ��ܼ��첿��û�����ݣ��ζ�������������
c����ƿ����Һ��ɫ�仯����ɫ��Ϊ�ۺ�ɫ���������µζ���Һ�����ڿ̶ȣ�
d��ʢ������Һ����ƿ�ζ�ǰ�ô���ҺҺ��ϴ��
e����ƿ��װ������ּ�����������ˮ��ζ���
f�����ú�����NaCl��NaOH�������Ʊ���Һ�����ζ��������ᣮ
���� ��1�����ݱ��е����ݣ���һ�����Ϊ14.30mL��ڶ��Ρ����������ϴ����Ե�һ������żȻ���ϴ�Ӧȥ�����õڶ��κ͵����εľ�ֵ��
��2�����ݷ���ʽ5Fe2++8H++MnO-4�T5Fe3++Mn2++4H2O�����ø�����ص����ʵ����ɼ������Ԫ�ص�������
��3���Լ�1���ܽ����ã����ᡢϡ������ɣ����Լ�2���������ᣬ�ױ��������������Ӱ��ʵ��ⶨ��
��4���ñ�Һ��ϴ�ζ���ʱ�����ע�������ϴҺ�ӵζ����¶˷ų��������Ǵ��Ͽڵ�����KMnO4��Һ����ʽ�ζ���ʢ�ţ��ζ�ʱ��ת������
��5��a����ʽ�ζ�����װ��Һǰδ�ô���������Һ��ϴ����Һ��ϡ�ͣ�
b����ʼʵ��ʱ��ʽ�ζ��ܼ��첿��û�����ݣ��ζ������������ݣ����ñ�Һ���ƫ��
c����ƿ����Һ��ɫ�仯����ɫ��Ϊ�ۺ�ɫ���������µζ���Һ�����ڿ̶ȣ���ʱ��Һ�������㣬��ñ�Һ���ƫС��
d��ʢ������Һ����ƿ�ζ�ǰ�ô���ҺҺ��ϴ����ȡ�õĴ���Һƫ�ࣻ
e����ƿ��װ������ּ�����������ˮ��ζ�����ʵ����û��Ӱ�죻
f�����ú�����NaCl��NaOH�������Ʊ���Һ�����ζ��������ᣬ���Һ��Ũ��ƫС����ȥ�ı�Һ���ƫ��
��� �⣺��1�����ݱ��е����ݣ���һ�����Ϊ14.30mL��ڶ��Ρ����������ϴ����Ե�һ������żȻ���ϴ�Ӧȥ�����õڶ��κ͵����εľ�ֵ��������ͬѧ�ڼ���ʱȡ��KMnO4���������Ϊ$\frac{12.22+11.98}{2}$mL=12.0mL��
�ʴ�Ϊ��12.0��
��2�����ݷ���ʽ5Fe2++8H++MnO-4�T5Fe3++Mn2++4H2O���ζ������ĵĸ�����ص����ʵ���Ϊ12.0mL��10-3��0.020mol/L=2.4��10-4mol��������Ʒ��Ԫ�ص�����Ϊ2.4��10-4mol��5��$\frac{1000}{20}$��56g/mol=3.36g���������ĺ�����$\frac{3.36g}{10g}$��100%=33.6%��
�ʴ�Ϊ��33.6%��
��3���Լ�1���ܽ����ã����ᡢϡ������ɣ����Լ�2���������ᣬ�ױ��������������Ӱ��ʵ��ⶨ�����ʵ���е��Լ�2���ͬѧ��Ƶ�ʵ���е��Լ�1��������ϡ���ᣬ
�ʴ�Ϊ��C��
��4���ñ�Һ��ϴ�ζ���ʱ�����ע�������ϴҺ�ӵζ����¶˷ų��������Ǵ��Ͽڵ������������������ʽ�ζ����м���1-2mLKMnO4��Һ�����ζ��ܺ������ת������KMnO4��Һ�����ζ����ڱڣ�������ŵ���ϴҺ���ظ�����2-3�Σ�KMnO4��Һ����ʽ�ζ���ʢ�ţ��ζ�ʱ������ת������ֻ��ͼA���ϣ�
�ʴ�Ϊ������ʽ�ζ����м���1-2mLKMnO4��Һ�����ζ��ܺ������ת������KMnO4��Һ�����ζ����ڱڣ�������ŵ���ϴҺ���ظ�����2-3�Σ�A��
��5��a����ʽ�ζ�����װ��Һǰδ�ô���������Һ��ϴ����Һ��ϡ�ͣ�ȡ�õ���Һƫ�٣�����ʵ����ƫ�ͣ�
b����ʼʵ��ʱ��ʽ�ζ��ܼ��첿��û�����ݣ��ζ������������ݣ����ñ�Һ���ƫС������ʵ����ƫ�ͣ�
c����ƿ����Һ��ɫ�仯����ɫ��Ϊ�ۺ�ɫ���������µζ���Һ�����ڿ̶ȣ���ʱ��Һ�������㣬��ñ�Һ���ƫС������ʵ����ƫ�ͣ�
d��ʢ������Һ����ƿ�ζ�ǰ�ô���ҺҺ��ϴ����ȡ�õĴ���Һƫ�࣬����ʵ����ƫ�ߣ�
e����ƿ��װ������ּ�����������ˮ��ζ�����ʵ����û��Ӱ�죻
f�����ú�����NaCl��NaOH�������Ʊ���Һ�����ζ��������ᣬ���Һ��Ũ��ƫС����ȥ�ı�Һ���ƫ����ʵ����ƫ�ߣ�
��ѡdf��
���� ���⿼�����ʵĺ�����̽��ʵ�飬Ϊ��Ƶ���㣬���շ����Ļ�ѧ��Ӧ��ʵ�����Ϊ���Ĺؼ������ط������������������Ŀ��飬ע����Ϣ����ѧ֪ʶ�Ľ�ϣ���Ŀ�ѶȲ���

 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�| A�� | CH3COOH��l�� | B�� | CH3COOH��g�� | C�� | CO��g�� | D�� | CO2��g�� |
| A�� | ̼����������ᷴӦ���ɶ�����̼�����ƣ��������������е��ᷴӦ�������ɶ������� | |
| B�� | Ũ������п������Ӧ���Ȳ�����������������������ƣ�Ũ���������н�����Ӧ�����Ȳ������������������ | |
| C�� | Ũ���ᡢŨ�����ڿ����з��ã���Һ��������ᣮ���ƣ�����Ũ���ڿ����з��ã���Һ����������� | |
| D�� | ƫ��������Һ����̼��������Һ������Ӧ�����������������ƣ���ƫ��������Һ��ͨ�������̼Ҳ�������������� |
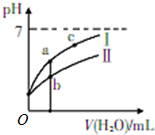 ��֪���Ϊ25��ʱijЩ����ĵ���ƽ�ⳣ������ͼ��ʾ����ʱ��ϡ��CH3COOH��HClO�������ϡ��Һʱ����ҺpH���ˮ���ı仯��
��֪���Ϊ25��ʱijЩ����ĵ���ƽ�ⳣ������ͼ��ʾ����ʱ��ϡ��CH3COOH��HClO�������ϡ��Һʱ����ҺpH���ˮ���ı仯��| CH2COOH | HClO | H2CO2 |
| Ka=1.8��10-5 | Ka=3.0��10-8 | Ka1=4.4��10-7Ka2=4.7��10-11 |
��2��a��b��c�����У�ˮ�ĵ���̶��ɴ�С��˳����c��a��b���ñ�ű�ʾ����
��3��25��ʱ��NaClO��Һ��ˮ��ƽ�ⳣ��Kh=3.3��10-7molL-1��
��4��25��ʱ��NaHCO3��Һ����ˮ��ƽ�⣬д����ˮ������ӷ���ʽHCO3-+H2O�PH2CO3+OH-
��5��0.1mol/L Na2CO3��Һ��c��OH��-c��H+��=c��HCO3-��+2c��H2CO3�������ú�c��HCO${\;}_{3}^{-}$����c��H2CO3���Ĺ�ϵʽ��ʾ����
| A�� | 1mol�һ���-C2H5���к��еĵ�����Ϊ16NA | |
| B�� | 8g CH4���10NA������ | |
| C�� | ��״����22.4L�ȷ£����ȼ��飩�й��ۼ���ĿΪ4NA | |
| D�� | 28g��ϩ����ϩ����ϩ�Ļ�����壬��̼ԭ����Ϊ2NA |