��Ŀ����
9����������Ҫ��һԪ�ᣬ���л�������Ӧ�ж���Ӧ�ã���1����������Ӧ��ʵ���У����ᡢ�Ҵ���������ƽ��ʱ�������������������±����ɱ��������Ʋ⣬��ֵx�ķ�Χ��1.57��x��1.76��
| ��Ӧ | �Ҵ���mol�� | ���ᣨmol�� | ����������mol�� |
| 1 | 2 | 2 | 1.33 |
| 2 | 3 | 2 | 1.57 |
| 3 | 4 | 2 | x |
| 4 | 5 | 2 | 1.76 |
����������м������������ƹ��壬��ʱ��Һ��$\frac{c��{H}^{+}��}{c��C{H}_{3}COOH��}$����С���������С�����䡱����
����������м���ϡNaOH��Һ��ʹ��ǡ����ȫ��Ӧ��������Һ��pH��7���������������=�����������ӷ���ʽ������ԭ��CH3COO-+H2O?CH3COOH+OH-��
����������м���pH=11��NaOH��Һ���Ҷ��ߵ������Ϊ1��1����������Һ�и����ӵ����ʵ���Ũ���ɴ�С��˳����c��CH3COO-����c��Na+����c��H+����c��OH-����
�ܵ������Ũ��Ϊ0.1mol/Lʱ����ʱ����ĵ��볣��ԼΪ1��10-5��
���� ��1�����ݷ�Ӧ��Ũ�ȶԻ�ѧƽ���Ӱ�죻
��2���ٸ��ݴ����ƹ����ƽ��CH3COOH?CH3COO-+H+��Ӱ�죻
�ڸ���ǿ��������Ҫ����ˮ��ʼ��ԣ�
�۸��ݴ����������Һ�еijɷ�Ϊ����ʹ����ƣ����ݴ���ĵ���̶ȴ��ڴ����Ƶ�ˮ��̶������
�ܴ����Ũ��Ϊ0.1mol/Lʱ��pH=3���ݴ˷�����
��� �⣺��1��������������ʵ�����ͬ�����£������Ҵ������ʵ���ƽ�������ƶ����������������ʵ������ӣ������Ҵ������ʵ���ƽ�������ƶ����������������ʵ������٣�����1.57��X��1.76���ʴ�Ϊ��1.57��X��1.76��
��2����������ƹ���������CH3COO-��c��CH3COO-������ʹ��ƽ��CH3COOH?CH3COO-+H+�����ƶ���c��H+����С��c��CH3COOH����������$\frac{c��{H}^{+}��}{c��C{H}_{3}COOH��}$��С���ʴ�Ϊ����С��
��������м���ϡNaOH��Һ��ʹ��ǡ����ȫ��Ӧ�������˴����ƣ�������ˮ��ʼ��ԣ����ӷ���ʽΪCH3COO-+H2O?CH3COOH+OH-��
�ʴ�Ϊ������CH3COO-+H2O?CH3COOH+OH-��
��pH=3�Ĵ����pH=11��NaOH��Һ�����Ϊ1��1��Ӧʱ�����������Һ�еijɷ�Ϊ����ʹ����ƣ���Һ�����ԣ������ĵ���̶ȴ��ڴ����Ƶ�ˮ��̶ȣ�����c��CH3COO-����c��Na+����c��H+����c��OH-����
�ʴ�Ϊ��c��CH3COO-����c��Na+����c��H+����c��OH-����
�ܴ����Ũ��Ϊ0.1mol/Lʱ��pH=3��K=$\frac{[{H}^{+}]•[C{H}_{3}CO{O}^{-}]}{[C{H}_{3}COOH]}$=$\frac{0.001��0.001}{0.1-0.001}$=1��10-5���ʴ�Ϊ��1��10-5��
���� ������Ҫ�����˵���ƽ�⡢ˮ��ƽ��͵���ƽ�ⳣ�������㣬�ѶȲ����ݻ�ѧƽ��֪ʶ�����

 һ���㶨ϵ�д�
һ���㶨ϵ�д� ��У��ҵ��ϵ�д�
��У��ҵ��ϵ�д� ���ɶ���ܲ��¿�ֱͨ��Уϵ�д�
���ɶ���ܲ��¿�ֱͨ��Уϵ�д�| A�� | CH3CH2CH2CH3��CH3CH=CHCH3 | B�� | CH3CH=CHCH3�� CH3C��CCH3 | ||
| C�� | CH3CH=CHCH3��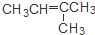 | D�� | CH3CH2CH2OH��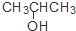 |
| A�� |  ��ͼ�ɱ�ʾˮ�ֽ�����е������仯 ��ͼ�ɱ�ʾˮ�ֽ�����е������仯 | |
| B�� | ��2C��s��+O2��g��=2CO��g����H=-221.0 kJ/mol����̼��ȼ����Ϊ110.5 kJ/mol | |
| C�� | ��Ҫ���ȵķ�Ӧһ�������ȷ�Ӧ���������ܷ����ķ�Ӧһ���Ƿ��ȷ�Ӧ | |
| D�� | ��֪�����ڷ�Ӧ��H2��g��+Cl2��s��=2HCl ��g����H=-a kJ/mol�� �� 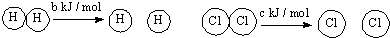 ��a��b��c�������㣬��Ͽ�1 mol H-Cl�����������Ϊ-a-b-c ��a��b��c�������㣬��Ͽ�1 mol H-Cl�����������Ϊ-a-b-c |
| A�� | 100 mL 2 mol/L������п��Ӧʱ����п�۴���п�����������������ʲ��� | |
| B�� | ���ڷ�Ӧ2CO+2NO?N2+2CO2��ʹ�ú��ʵĴ�����CO���������ʺ��������ʶ��ӿ� | |
| C�� | ��������Ĵ�������һ�����ȷ�Ӧ�������¶ȣ���Ӧ���ʼ��� | |
| D�� | ����Ƭ��ϡ���ᷴӦ��ȡ����ʱ����ϡ�����ΪŨ������Լӿ��������������� |
| A�� | ZnC2ˮ���������� | B�� | Al4C3ˮ�����ɱ�Ȳ | ||
| C�� | Mg2C3ˮ�����ɱ�Ȳ | D�� | Li2C2ˮ��������ϩ |
| A�� | ����������ȩ������������ȫȼ��ʱ����������������� | |
| B�� | ����ˮ������ղ����ڼ��������¿�������������ͭ��Ӧ����ש��ɫ���� | |
| C�� | ���ñ���̼������Һ��ȥ���������е����� | |
| D�� | 2-��-2-���������������ƵĴ���Һ�м��ȿ����ɼ���ϩ |
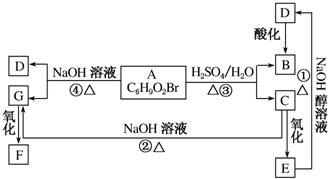 �л���A��B��C��D��E��F��G���ϵ��ͼ��ʾ����֪F�����к���������ͬ�Ĺ����ţ�5.2gF����100mL1mol•L-1��NaOH��Һǡ����ȫ�кͣ�D�ķ���ʽΪC3H3O2Na��E�ķ����к����Ȼ���
�л���A��B��C��D��E��F��G���ϵ��ͼ��ʾ����֪F�����к���������ͬ�Ĺ����ţ�5.2gF����100mL1mol•L-1��NaOH��Һǡ����ȫ�кͣ�D�ķ���ʽΪC3H3O2Na��E�ķ����к����Ȼ���