��Ŀ����
10��6��ij���οɱ�ʾΪ��[xFeSO4•yAl2 ��SO4��3•24H20]����Ħ������Ϊ 926g•mol-1�����������Ʊ���Ч�ĸ�����Ч������������ɿ�ͨ������ʵ��ⶨ����ȡһ����������������Ʒ��ȷ���100mL��ҺA��
����ȡ25.00mL��ҺA�����������ữ��BaCl2��Һ��������ȫ�����ˡ�ϴ�ӣ��������� �أ��õ���ɫ����4.660g��
������ȡ25.00mL��ҺA���μ�����ϡ���ᣬ��0.1000mol•L-1 KMnO4��Һ�ζ����յ㣬����Mn2+������KMnO4��Һ10.00mL��
��1����25.00mLʽ���У�n��S042-��=2.000��10-2mol��n��Fe2+��=5.000��10-3mol
��2���������Ļ�ѧʽΪFeSO4•Al2��SO4��3•24H2O��
���� ��1����ȡ25.00mL��ҺA�����������ữ��BaCl2��Һ��������ȫ�����ˡ�ϴ�ӡ����������أ��õ���ɫ����4.660gΪBaSO4�����ʵ���=$\frac{4.660g}{233g/mol}$=0.02mol���õ�������������ʵ�������MnO4-+5Fe2++8H+=Mn2++5Fe3++4H2O�������������ӱ����������Һ����Ϊ�����ӷ�Ӧ�Ķ�����ϵ����õ���
��2������100ml��Һ�к�����������ӡ������������ʵ����õ�xy���ʵ���֮�ȵõ���ѧʽ��
��� �⣺��1��ȡ25.00mL��ҺA�����������ữ��BaCl2��Һ��������ȫ�����ˡ�ϴ�ӣ����������أ��õ���ɫ����0.4660g����������������غ㣬��������ӵ����ʵ��������ᱵ�����ʵ�������ȵģ�����$\frac{4.660g}{233g/mol}$=0.02mol=2.000��10-2mol��
ȡ25.00mL��ҺA���μ�����ϡ���ᣬ��0.10 0mol•L-1 KMnO4��Һ�ζ����յ㣬����Mn2+������KMnO4��Һ10.00mL�������Ի����£�����������ӿ��Խ�������������Ϊ���������ӣ���
MnO4-+5Fe2++8H+=Mn2++5Fe3++4H2O��
1 5
0.100mol/L��10��10-3L n
�����������ӵ����ʵ���Ϊ��n=5��0.100mol/L��10��10-3 L=5��10-3mol��
�ʴ�Ϊ��2.000��10-2��5.000��10-3��
��2��100mL��Һ�к�����������ӵ����ʵ���Ϊ��n��SO42-��=2.000��10-2mol��$\frac{100}{25}$=0.08mol��
�����������ӵ����ʵ���Ϊ��n��Fe2+��=5��10-3mol��$\frac{100}{25}$=0.02mol��
�����Ʒ�к���xFeSO4•y��NH4��2SO4•6H2O�����ʵ���Ϊn��
��n��SO42-��=nx+ny=0.08��n��Fe2+��=nx=0.04��
�����ɵã�x��y=1��1��
���Ըû�����Ļ�ѧʽΪ��FeSO4•Al2��SO4��3•24H2O��
�ʴ�Ϊ��FeSO4•Al2��SO4��3•24H2O��
���� ���⿼�������ʵ����ļ��㡢���ӻ�ѧʽ�ļ��㣬��Ŀ�Ѷ��еȣ���ȷ������Ӧ��ʵ��Ϊ���ؼ�������������ϴ�ֿ�����ѧ���ķ�����������������ѧ����������

| A�� | V1��V2 | B�� | V1��V2 | C�� | V1=V2 | D�� | V1��V2 |
| A�� | �����ȶ��ռ������壬����ζ�����ռ��� | |
| B�� | ����ʪ�ĵ���-KI��ֽ�ӽ�ƿ�ڣ���ֽ���������ռ��� | |
| C�� | ����ʪ�ĺ�ɫʯ����ֽ����ƿ�ڣ���ֽ��ɫ�����ռ��� | |
| D�� | ����˿�쵽ƿ�ڣ���˿ȼ��˵�����ռ��� |
| A�� | 11.2LCl2ͨ����������������Һ�г�ַ�Ӧ��ת�Ƶĵ���������0.5NA | |
| B�� | 1 mol Al3+��ȫˮ���������������������ӵ���ĿΪNA | |
| C�� | ���³�ѹ�£�32 g O2-���������ӵ���ĿΪ17NA | |
| D�� | ��״���£�11.2 L���к��з��ӵ���ĿΪ0.5 NA |
| A�� | �Ȼ�����Һ�м��������ˮ��Al3++3NH3•H2O�TAl��OH��3��+3 NH4+ | |
| B�� | ����ˮ�ķ�Ӧ��Na+2H2O�TNa++2OH-+H2�� | |
| C�� | ͭƬ��ϡ����ķ�Ӧ��Cu+NO3-+4H+�TCu2++NO��+2H2O | |
| D�� | ��������������������Һ��Al+2OH-�TAlO2-+H2�� |
| A�� | ����������Һ�У�NH${\;}_{4}^{+}$��Na+��HSO${\;}_{3}^{-}$��Cr2O${\;}_{7}^{2-}$ | |
| B�� | c��H+��=10-13mol•L-1����Һ�У�Cl-��Br-��S2O${\;}_{3}^{2-}$��Na+ | |
| C�� | ��ʹpH��ֽ������ɫ����Һ�У�K+��Na+��AlO${\;}_{2}^{-}$��HCO${\;}_{3}^{-}$ | |
| D�� | ����Al�ܷų�H2����Һ�У�NO${\;}_{3}^{-}$��K+��SO${\;}_{4}^{2-}$��Mg2+ |
 �����仯���ﳣ�����Ͻ𡢴������缫���ϣ���֪����Ԫ����+2��+3�۵�����������������������+2�۵���ʽ���ڣ�
�����仯���ﳣ�����Ͻ𡢴������缫���ϣ���֪����Ԫ����+2��+3�۵�����������������������+2�۵���ʽ���ڣ���1������ϡ���ᷴӦ�ų�NO����Ӧ�Ļ�ѧ����ʽ��3Ni+8HNO3=3Ni��NO3��2+2NO��+4H2O��
��2��Ni2O3��һ����Ҫ�ĵ�ز��ϣ�
���������طŵ�ʱ�ܷ�ӦΪ��Fe+Ni2O3+3H2O=Fe��OH��2+2Ni��OH��2
��طŵ�ʱ��������ӦΪFe+2OH--2e-=Fe��OH��2��
��س��ʱ��������ӦΪ2Ni��OH��2+2OH--2e-=Ni2O3+6H2O��
���ڼ��������£������ص��ܷ�ӦΪ��3Ni2O3+LaNi5H6+3H2O$?_{���}^{�ŵ�}$LaNi5+6Ni��OH��2���õ�طŵ�ʱ�������缫��ӦΪLaNi5H6+6OH--6e-=LaNi5+6H2O
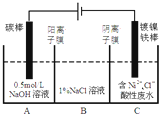
��3����ҵ�ϵ�ⷨ�����������Է�ˮ���õ�����Ni��ԭ����ͼ��ʾ��
��֪��
��Ni2+����������Һ�з���ˮ��
�������ԣ�Ni2+����Ũ�ȣ���H+��Ni2+����Ũ�ȣ�
����˵������ȷ����BD
A��̼���Ϸ����ĵ缫��Ӧ��4OH--4e- O2��+2H2O
B���������У�B����NaCl��Һ�����ʵ���Ũ�Ƚ����ϼ���
C��Ϊ�����Ni�IJ��ʣ�����������Ҫ���Ʒ�ˮpH
D������ͼ��������Ĥȥ������A��B���Һϲ������ⷴӦ�ܷ���ʽ�������ı�
��4��ij�����÷ϵĺ�����������Ҫ��Ni��������һ������Al��Fe��SiO2�����������������£�

��֪�����������У���������������ȫ����ʱ��pH���±���
| ���� | Al��OH��3 | Fe��OH��3 | Fe��OH��2 | Ni��OH��2 |
| pH | 5.2 | 3.2 | 9.7 | 9.2 |
������b�����ɷݵĻ�ѧʽΪAl��OH��3��Fe��OH��3��
��������Һ����ΪpH=6ʱ������bkgNi��OH��2�����������ҺB�Ĺ�����������ʧ��Ϊ3%��������������������ʧ��Ϊ5%��akg�ϴ�������Ni70%���������������յõ�������������Ϊ��a��70%��97%+$\frac{59}{93}$b����95%kg�������ʽ����
