��Ŀ����
����Ŀ����Zn(��Ҫ����Fe��Al��Pb����)����������ȡH2�����������Һ�Ʊ�����п����(ZnSO4��7H2O)��Al2O3��Fe2O3������������

��֪Al3����Fe3����Zn2��������������ȫ������pH�ֱ�Ϊ5.2��4.1��8.5��ZnSO4��7H2O����������ˮ���绯���ش�����������
(1)����pH��2��Ŀ����______________________������pH��2���ɼ���________________(�ѧʽ)��
(2)д�����ɳ���3�Ļ�ѧ����ʽ��_____________________________________��
(3)����Ũ��ZnSO4��Һ���ּ�����Ĥʱ��Ҫֹͣ���ȵ���Ҫԭ����_______________________________��
(4)
ijͬѧ����ͼ��ʾ��װ�ó�����

���йس��˵�˵����ȷ����________(����ĸ)��
A�����˵�Ŀ����Ҫ�ǵõ��ϸ���ij���
B����ֽ��ֱ��Ӧ��С��©���ھ������ܸ�סȫ��С��
C��ͼ����һ������
D�����˽�����������ƿ��֧�ܿڵ�����Һ
��������ϴ�ӳ����ľ��������____________________________________________________��
(5)Ϊ�õ������ZnSO4��7H2O��Ʒ��ѡ����﷽����________(����ĸ)��
A�����Ⱥ��
B����ŨH2SO4����
C���þƾ�ϴ��
D���ڿ�������Ȼ����
���𰸡� ����Zn2��ˮ�⣬Zn2����2H2O![]() Zn(OH)2��2H�� H2SO4 NaHCO3��NaAlO2��H2O===Al(OH)3����Na2CO3 ��ֹʧȥ�ᾧˮ B �ȹ�Сˮ��ͷ������ϴ�Ӽ����������ʹϴ�Ӽ�����ͨ�������� C
Zn(OH)2��2H�� H2SO4 NaHCO3��NaAlO2��H2O===Al(OH)3����Na2CO3 ��ֹʧȥ�ᾧˮ B �ȹ�Сˮ��ͷ������ϴ�Ӽ����������ʹϴ�Ӽ�����ͨ�������� C
��������(1)����pH��5.2ʱ����������Ҫ�ɷ���Al(OH)3��Fe(OH)3����Һ�к���ZnSO4 ��������ҺpH��2��������ZnSO4ˮ�⣬��ֹ����Zn(OH)2��ͬʱΪ�˱����������ʣ�Ӧ��ʹ��H2SO4��(2)����Al(OH)3��Fe(OH)3��NaOH����������NaAlO2��Fe(OH)3���μӷ�Ӧ�����˺���Һ�к���NaAlO2��NaOH������NaHCO3��Ӧ���ɵij�����Al(OH)3����ѧ����ʽ��NaHCO3��NaAlO2��H2O===Al(OH)3����Na2CO3��(3)����ZnSO4��7H2O�����绯������Ũ��ZnSO4��Һʱ���ᾧ�õ��ľ������ֽ�ʧȥ�ᾧˮ��(4)�ٳ������ڹ��ˣ��������ٶȱȽϿ죬���ҹ��˽ϳ��ף�ѡ��A����Ϊ�˷�ֹ��ֽ��Ե���������©�������ȫ���ϣ����Գ��˵���ֽҪ��С��©���ھ������DZ����ס����С�ף�ѡ��B��ȷ��ԭװ��ͼ�а�ȫƿ��������ƿ�ĵ��ܲ������밲ȫƿ��̫��������©���¶�б��Ӧ�Գ����죬ѡ��C����ʵ���������Һ������ƿ�Ͽڵ�����ѡ��D����ѡB����ϴ�ӳ���ʱ���ȵ�С������ѹ����ڳ���©���м���ˮ��û��������(5)ZnSO4��7H2O�����绯�����Ⱥ�ɻᵼ�¾���ʧȥ�ᾧˮ��A����Ũ�������ǿ�ҵ���ˮ�ԣ���Ũ�������Ҳ��ʹ����ʧȥ�ᾧˮ��B���ƾ��ܹ��ܽ�ˮ�������ᵼ�¾���ʧȥ�ᾧˮ��ϴ�Ӻ�ƾ������¾ͺ����ӷ���C��ȷ����������Ȼ����Ҳ��绯��D����ѡC��

 ��У����ϵ�д�
��У����ϵ�д�����Ŀ��ú������Һ�����ִ���Դ��ҵ���ص㿼�ǵ���Դ�ۺ����÷������������������Ϊ��ú����ˮú��������ǰ�Ƚ����е�Һ������Ϊ��ú����CH3OH����֪�Ʊ��״����йػ�ѧ��Ӧ��ƽ�ⳣ��������
��CO2(g)��3H2(g) ![]() CH3OH(g)��H2O(g) ��H1����90.8 kJ��mol��1
CH3OH(g)��H2O(g) ��H1����90.8 kJ��mol��1
��CO(g)��H2O(g) ![]() CO2(g)��H2(g) ��H2����41.2 kJ��mol��1
CO2(g)��H2(g) ��H2����41.2 kJ��mol��1
��CO(g)��2H2(g) ![]() CH3OH(g)����H3
CH3OH(g)����H3
850 ��ƽ�ⳣ���ֱ�ΪK1��160��K2��243��K3��160���״����������ᷴӦ������CH3OH(l)��CH3COOH(l) ![]() CH3COOCH3(l)��H2O(l)��
CH3COOCH3(l)��H2O(l)��
(1)��Ӧ��H3��____________�������Ϸ�Ӧ��K�ı���ʽ________________��
(2)��CO�ϳɼ״�ʱ�������йظ÷�Ӧ��˵����ȷ����________(����ĸ)��
A���������������������������ڵ�ѹǿ�������仯������淴Ӧ�ﵽƽ��
B��һ����������H2 ������������CO���������ʵ�2��ʱ�����淴Ӧ�ﵽƽ��
C��ʹ�ú��ʵĴ��������̴ﵽƽ���ʱ�䲢���CH3OH �IJ���
D��ij�¶�������2 mol CO��6 mol H2 ����2 L�ܱ�����������ַ�Ӧ���ﵽƽ��������c(CO)��0.2 mol��L��1����CO��ת����Ϊ80%
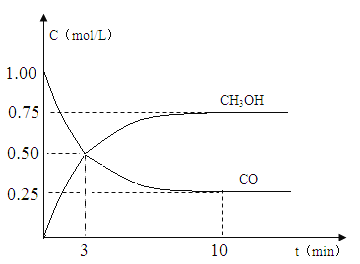
(3)850 ��ʱ�����ܱ������н��з�Ӧ������ʼʱֻ����CO2��H2����Ӧ10 min���ø���ֵ�Ũ�����±����Ƚ������淴Ӧ�����ʵĴ�С��v��________(����������������������)v������ʱ����ڷ�Ӧ����v(H2)��____________________________________________________��
���� | H2 | CO2 | CH3OH | H2O |
Ũ��/mol��L��1 | 0.2 | 0.2 | 0.4 | 0.4 |
(4)��һ��������3 L�����ܱ�������������һ������H2��CO2��������Ӧ����ʵ���÷�Ӧ���ڲ�ͬ��ʼͶ����������Ӧ��ϵ��CO2��ƽ��ת�������¶ȵĹ�ϵ��������ͼ1��ʾ��
![]()

��H2��CO2����ʼ��Ͷ������A��B���ַ�ʽͶ��
A��n(H2)��3 mol��n(CO2)��1.5 mol��
B��n(H2)��3 mol��n(CO2)��2 mol����������������Ͷ�뷽ʽ________(��A��B��ʾ)��
�����¶�Ϊ500 K��������������A��ʽ����3 mol H2��1.5 mol CO2���÷�Ӧ10 minʱ�ﵽƽ�����ڴ���������ϵͳ��CH3OH��Ũ���淴Ӧʱ��ı仯������ͼ2��ʾ������Ӧʱ��ﵽ3 minʱ��Ѹ�ٽ���ϵ�¶�����600 K������ͼ2�л���3��10 min��������CH3OHŨ�ȵı仯����������_____________________