��Ŀ����
����Ŀ���������⡿���ܼ����Ѿ���Ϊȫ���Ĺ�ʶ���㽭ʡ��ԭ�������Ҵ����͵Ļ����ϣ���ʼ�Ե�״����ͣ���������������һ�����ļ״��������ݼ������ݷ�����Ϊ��������ȫ�е�100������������ȫ��ʹ�ü״����ͣ�һ�����ܼ����к����壨һ����̼���ŷŽ���100��֡��״�������ú�������������ɵ�CO��H2���Ʊ���CO+2H2CH3OH ��
�����ͼʾ�ش����У�
��1�����ڸ��Է���Ӧ������˵���У���ȷ���� ������ĸ����
A����H>0����S>0 B����H>0����S<0
C����H<0����S<0 D����H<0����S>0
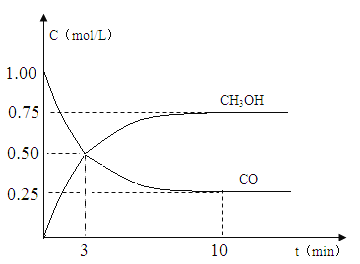
��2���ֽ�������ʵ�飬�����Ϊ1L���ܱ������У�����1molCO��3molH2�����CO��CH3OH(g)��Ũ����ʱ��仯��ͼ��ʾ���ӷ�Ӧ��ʼ��ƽ�⣬CO��ƽ����Ӧ����v��CO��= mol/(Lmin)���÷�Ӧ��ƽ�ⳣ��K= ��
��3�����������£��ﵽƽ������д�ʩ����ʹn(CH3OH)/n(CO)������� ��
A�������¶� B������He�� C���ٳ���1molCO��3molH2 D��ʹ�ô���
��4������һ����ɱ���ܱ������г���1molCO��2molH2��1molCH3OH���ﵽƽ��ʱ��Ļ��������ܶ���ͬ��ͬѹ����ʼ��1.6�����˹����������ʾ�������̬������ƽ��ʱ��������ƽ��Ħ������= g/mol��
���𰸡���1�� C ��2�֣���2��0.075 ��2�֣� K=4/3 ��2�֣�
��3�� C ��2���� ��4�� 25.6 ��2����
��������
�����������1���÷�ӦΪ���������С�ķ�Ӧ��������S<0����H-T��S<0����Ӧ�Է�������������H<0����C����2����ͼ��֪��10min��ƽ����CO��Ũ��Ϊ0.25mol/L������c��CO��=��1.00-0.25��mol/L=0.75 mol/L����ƽ��ʱc(CH3OH)= c��CO��=0.75 mol/L��ƽ��ʱc(H2)=3 mol/L-2��c��CO��=1.5 mol/L����v��CO��=0.75 mol/L��10min=0.075 mol/(Lmin)���÷�Ӧ��ƽ�ⳣ��K= ![]() =4/3����3�� A������ӦΪ���ȷ�Ӧ�������¶�ƽ�������ƶ���n(CH3OH)/n(CO)��С��������B������He�������º��������¸�����Ũ�Ȳ�����ƽ�ⲻ�ƶ���n(CH3OH)/n(CO)������������C���ٳ���1molCO��3molH2����ЧΪ����ѹǿ��ƽ�������ƶ���n(CH3OH)/n(CO)��������ȷ��D��ʹ�ô���ֻ�ӿ췴Ӧ��������Ӱ��ƽ���ƶ���n(CH3OH)/n(CO)������������4�� ����1molCO��2molH2��1molCH3OH����ʼ��ƽ��Ħ������
=4/3����3�� A������ӦΪ���ȷ�Ӧ�������¶�ƽ�������ƶ���n(CH3OH)/n(CO)��С��������B������He�������º��������¸�����Ũ�Ȳ�����ƽ�ⲻ�ƶ���n(CH3OH)/n(CO)������������C���ٳ���1molCO��3molH2����ЧΪ����ѹǿ��ƽ�������ƶ���n(CH3OH)/n(CO)��������ȷ��D��ʹ�ô���ֻ�ӿ췴Ӧ��������Ӱ��ƽ���ƶ���n(CH3OH)/n(CO)������������4�� ����1molCO��2molH2��1molCH3OH����ʼ��ƽ��Ħ������![]() =16g/mol��ͬ��ͬѹ�£�������ܶ�֮�ȵ���Ħ������֮�ȣ�������ƽ��ʱ��������ƽ��Ħ������=16g/mol��1.6=25.6 g/mol��
=16g/mol��ͬ��ͬѹ�£�������ܶ�֮�ȵ���Ħ������֮�ȣ�������ƽ��ʱ��������ƽ��Ħ������=16g/mol��1.6=25.6 g/mol��

����Ŀ�����й��ڴ������������ʡ��ǵ���ʵ���ȷ���Ϊ(����)
������ | ����� | ����� | �ǵ���� | |
A | ���� | ���� | ���� | �ɱ� |
B | ��ˮ����� | ������Һ | ������ | �������� |
C | ���� | ���� | �� | ���� |
D | Ũ���� | ʳ��ˮ | �Ȼ�ͭ | ̼���� |
A. A B. B C. C D. D






