��Ŀ����
����Ŀ��
��1������ʱ��ˮ��c(H+)=c(OH-)����ˮ�м����������й������ʣ�
A.NaOH B.Na2SO4 C.NH4Cl
�Իش��������⣺
I.��Һ��H+��OH-Ũ�ȱ仯�����������С�����䡱��
II. c(H+)��c(OH-)��С�Ƚϣ��������������=����
III.��Һ������ԣ�����ԡ������ԡ������ԡ���
�밴����Ҫ�����������������
A.NaOH | a Na2SO4 | C.NH4C1 | |
c(H+) | ���� | ��________ | ���� |
c(OH-) | ��_______ | ���� | ��_____ |
c(H+)��c(OH-)�Ĵ�С�Ƚ� | ��_______ | = | > |
��Һ������� | ���� | ��_______ | ���� |
(2)��֪25��ϳɰ���Ӧ�У�1 mol N2��ȫת��ΪNH3ʱ�ͷŵ�����Ϊ92.4 kJ��X�ֽ�1 molN2��3 mol H2�������2 L�ܱ������У���Ӧ���е�2 sĩ���NH3Ϊ0.4mol���Իش�
�ٸ÷�Ӧ���Ȼ�ѧ������ʽΪ ________________________��
�ڸ÷�Ӧ�ﵽƽ������{�¶�ƽ����_____(��������桱)��Ӧ�����ƶ�������ѹǿƽ����_____ (��������桱)��Ӧ�����ƶ����������ƽ��________(��ܡ����ܡ�)�ƶ���
��ǰ2 s��v(H2)Ϊ_____________��
���𰸡� ���� ���� ���� < ���� N2(g) +3H2(g) ![]() 2NH3(g) ����H=-92.4 kJ/mol �� �� ���� 0.15mol/(L��s)
2NH3(g) ����H=-92.4 kJ/mol �� �� ���� 0.15mol/(L��s)
��������(1)����ʱ��ˮ��c(H+)=c(OH-)=10-7mol/L������NaOH�������c(OH-)����ˮ�ĵ���ƽ�����ƣ�����c(H+)��С������c(H+)��c(OH-)���ʢ�Ϊ����Ϊ��������Na2SO4���������Na2SO4��ǿ��ǿ������Σ�ˮ�ĵ���ƽ��û���ƻ�������Һ�е�c(H+)��c(OH-)�����䣬��Һ�����ԣ����ٲ��䣬�ݳ����ԣ�����NH4Cl���������NH4Cl��ǿ�������Σ��ܽ��ˮ�������OH-��������Һ��c(OH-)��С���ʢ�Ϊ���٣��ʴ�Ϊ���ٲ��䣻�����ۼ��٣��ܣ��������ԣ�
(2)����д�Ȼ�ѧ����ʽʱ����Ӧ���뷴Ӧ������ʵ����ɱ�������Ҫ��ע���ʵľۼ�״̬������1molN2��ȫת��ΪNH3ʱ�ͷŵ�����Ϊ92.4kJ�����Ȼ�ѧ����ʽΪ��N2(g)+3H2(g)�T2NH3(g)��H=-92.4kJ/mol���ʴ�Ϊ��N2(g)+3H2(g)�T2NH3(g)��H=-92.4kJ/mol��
�����ڴ˷�ӦΪ���ȷ�Ӧ���������¶ȣ�ƽ���������ȷ�Ӧ�����ƶ������淴Ӧ�����ƶ����˷�Ӧ����Ӧ���������������С�ķ�Ӧ��������ѹǿ��ƽ���������������С�ķ����ƶ���������Ӧ�����ƶ���ʹ�ô�����ƽ�ⲻ�ƶ����ʴ�Ϊ���棬�������ܣ�
�����ڷ�Ӧ���е�2sĩ���NH3Ϊ0.4mol����V(NH3)= 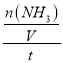 =
= =0.1mol/(Ls)����һ����Ӧ�и����ʵķ�Ӧ����֮�ȵ��ڼ�����֮�ȣ����У�v(H2)=
=0.1mol/(Ls)����һ����Ӧ�и����ʵķ�Ӧ����֮�ȵ��ڼ�����֮�ȣ����У�v(H2)= ![]() ��3=0.15mol/(Ls)���ʴ�Ϊ��0.15mol/(Ls)��
��3=0.15mol/(Ls)���ʴ�Ϊ��0.15mol/(Ls)��








