��Ŀ����
4��������X��һ�����ϣ��ɲ���ϩ��ױ�Ϊ��Ҫԭ�ϣ�������·�ߺϳɣ�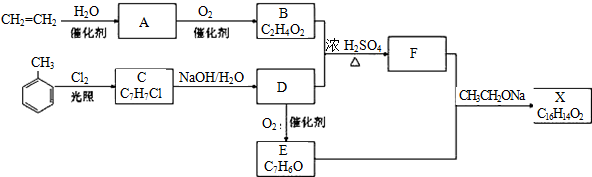
��֪��RX$\stackrel{NaOH/H_{2}O}{��}$ ROH��RCHO+CH3COOR��$\stackrel{CH_{3}CH_{2}ONa}{��}$RCH=CHCOOR��
��ش�
��1��E�������DZ���ȩ��
��2��B+D��F�Ļ�ѧ����ʽ
 ��
����3��X�Ľṹ��ʽ
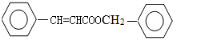 ��
����4�����ڻ�����X������˵����ȷ����AC��
A���ܷ���ˮ�ⷴӦ B������Ũ���ᷢ��ȡ����Ӧ
C����ʹBr2/CCl4��Һ��ɫ D���ܷ���������Ӧ
��5�����л�����������F��ͬ���칹�����BC��
A��
 CH2OCH2CH2CHO
CH2OCH2CH2CHOB��
 CH=CHCH2CH2CHO
CH=CHCH2CH2CHOC��CH2=CHCH=CHCH=CHCH=CHCOOH
D��
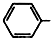 COOCH2CH2CH3��
COOCH2CH2CH3��
���� ��ϩ��ˮ�ڴ��������·����ӳɷ�Ӧ�����Ҵ�����A���Ҵ����Ҵ������������������ᣬ��B������ױ��ڹ�����������������������ȡ����Ӧ����CΪ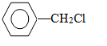 ��C���������Ƶ�ˮ��Һ�з�������ȡ����Ӧ����ôDӦΪ
��C���������Ƶ�ˮ��Һ�з�������ȡ����Ӧ����ôDӦΪ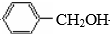 ����D�ܱ�����ΪE���ҽ�ϸ�������Ϣ��RCHO+CH3COOR��$\stackrel{CH_{3}CH_{2}ONa}{��}$RCH=CHCOOR�䣬��ôӦEΪ
����D�ܱ�����ΪE���ҽ�ϸ�������Ϣ��RCHO+CH3COOR��$\stackrel{CH_{3}CH_{2}ONa}{��}$RCH=CHCOOR�䣬��ôӦEΪ ���ݴ��ƶϵó�FΪ��
���ݴ��ƶϵó�FΪ��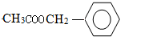 ����ôXΪ
����ôXΪ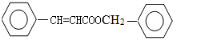 ���ݴ˴��⣮
���ݴ˴��⣮
��� �⣺��ϩ��ˮ�ڴ��������·����ӳɷ�Ӧ�����Ҵ�����A���Ҵ����Ҵ������������������ᣬ��B������ױ��ڹ�����������������������ȡ����Ӧ����CΪ ��C���������Ƶ�ˮ��Һ�з�������ȡ����Ӧ����ôDӦΪ
��C���������Ƶ�ˮ��Һ�з�������ȡ����Ӧ����ôDӦΪ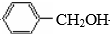 ����D�ܱ�����ΪE���ҽ�ϸ�������Ϣ��RCHO+CH3COOR��$\stackrel{CH_{3}CH_{2}ONa}{��}$RCH=CHCOOR�䣬��ôӦEΪ
����D�ܱ�����ΪE���ҽ�ϸ�������Ϣ��RCHO+CH3COOR��$\stackrel{CH_{3}CH_{2}ONa}{��}$RCH=CHCOOR�䣬��ôӦEΪ ���ݴ��ƶϵó�FΪ��
���ݴ��ƶϵó�FΪ��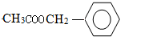 ����ôXΪ
����ôXΪ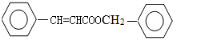 ��
��
��1�����ݷ�����֪��EΪ ������Ϊ����ȩ��
������Ϊ����ȩ��
�ʴ�Ϊ������ȩ��
��2��BΪ���ᣬDΪ���״������߷���������Ӧ�������ᱽ��������ѧ��Ӧ����ʽΪ�� ��
��
�ʴ�Ϊ�� ��
��
��3����������ķ�����֪��XΪ��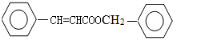 ��
��
�ʴ�Ϊ��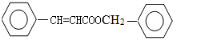 ��
��
��4��A��X�к����������ܷ���ˮ�ⷴӦ����A��ȷ��
B��X�к��б���������Ũ���ᷢ��ȡ����Ӧ����B����
C��X�к���̼̼˫������ʹBr2/CCl4��Һ��ɫ����C��ȷ��
D��X�в�����ȩ�������ܷ���������Ӧ����D����
��ѡAC��
��5��FΪ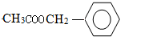 ������ʽΪ��C9H10O3��A�к���10��̼ԭ��������F����ͬ���칹�壬��A����B��C�ķ���ʽ��ΪC9H10O3������F�ṹ��ͬ������ͬ���칹�壻D��Hԭ�Ӹ���Ϊ12����F����ͬ���칹�壬��D����
������ʽΪ��C9H10O3��A�к���10��̼ԭ��������F����ͬ���칹�壬��A����B��C�ķ���ʽ��ΪC9H10O3������F�ṹ��ͬ������ͬ���칹�壻D��Hԭ�Ӹ���Ϊ12����F����ͬ���칹�壬��D����
��ѡBC��
���� ������Ҫ��������л���ĺϳ����л�����ƶϣ�������ճ����л�������������Լ�ץס������Ϣ�����ǹؼ����Ѷ��еȣ�ע���л������ŵ����ʵ�������ã�

 ǧ�������������ĩ�����Ծ�����ϵ�д�
ǧ�������������ĩ�����Ծ�����ϵ�д�| A�� | һ������ͬ������ | B�� | ������̼ԭ����һ����ͬ | ||
| C�� | �������һ������ͬһͨʽ | D�� | ���ܻ�Ϊͬ���칹�� |
| A�� | H��Cl | B�� | Na+ | C�� | 188O | D�� | O=C=O |
���Ҵ�ȼ�����ɵ�CO2�����Ǹ��л���ȼ�����ɵ�CO2������$\frac{2}{3}$��
�ڸ��л���ȼ������ˮ�������Ҵ�ȼ������ˮ������$\frac{2}{3}$��
�۶���������ͬ״̬�������������ͬ��ͨ�������жϸ��л����ǣ�������
| A�� | C4H9OH | B�� | CH2=CHCOOH | C�� | CH2=CHCHO | D�� | CH3CH2COOH |
| A�� | ��ɫ��Һ��K-��Na+��Cu2+��OH- | |
| B�� | ��ˮ�������c��OH-��=10-13mol/L����Һ�У�Na+��Ba2+��Cl-��Br+- | |
| C�� | ����Al�ܷų�H2����Һ�У�Cl-��HCO3-��SO42-��NH4+ | |
| D�� | �н϶�Fe3+����Һ�У�Na+��NH4+��SCN-��HCO3- |
| A�� | C6H6 | B�� | C2H4 | C�� | C2H5OH | D�� | C3H4 |
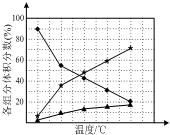 ��1��������N2H4����һ�ָ���ȼ�ϣ���ҵ�Ͽ������õ����������Ʊ�������
��1��������N2H4����һ�ָ���ȼ�ϣ���ҵ�Ͽ������õ����������Ʊ�������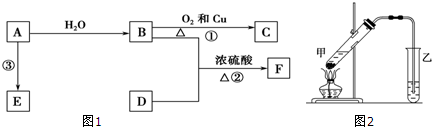
 ��B�о������ʵ���Ҫ�����ŵ�����Ϊ�ǻ���
��B�о������ʵ���Ҫ�����ŵ�����Ϊ�ǻ���
