��Ŀ����
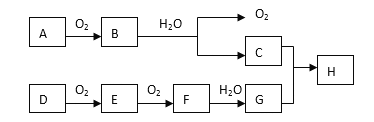
����Ŀ��ij��̬��A�ڱ�״���µ��ܶ�Ϊ1.25g/L�������������������һ�����ҵ�ʯ�ͻ�����չˮƽ��B��D���������г������л��D�ܸ�̼�����Ʒ�Ӧ��F����ζ������֮���ת����ϵ����ͼ��ʾ��

��1��A�ĽṹʽΪ__________________��B�й����ŵĵ���ʽΪ______________________�� D�й����ŵ�����Ϊ_________________��
��2����Ӧ���ķ�Ӧ������_________����Ӧ���Ļ�ѧ����ʽΪ_____________________��
��3����Ӧ����Cu�������������½��У���ʵ��IJ����ǽ�������ͭ˿���ھƾ����ϼ��ȣ���ͭ˿��Ϊ��ɫʱ��Ѹ�ٽ�����뵽װ��B���Թ��У���ͼ��ʾ�����ظ�����2��3�Σ��۲쵽��������_________________���÷�Ӧ�Ļ�ѧ����ʽΪ___________��

��4��D��̼��������Һ��Ӧ�����ӷ���ʽΪ_____________��
��5��B��D��Ũ�����������ʵ�ַ�Ӧ����ʵ��װ������ͼ��ʾ��
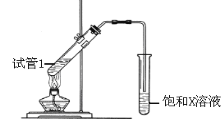
�Թ�1��װҩƷ��˳��Ϊ��ȡ__________������B������D������Ũ����������ͬ�����ټ���_______________��������____________��ͼ��X�Ļ�ѧʽΪ___����������____________________________��Ũ�����������_________________________���÷�Ӧ�Ļ�ѧ����ʽΪ_____________________________��
���𰸡�![]()
![]() �Ȼ� �ӳ� CH2=CH2 + Br2��CH2BrCH2Br ͭ˿�ɺ�ɫ���ɫ�������̼�����ζ 2CH3CH2OH+O2
�Ȼ� �ӳ� CH2=CH2 + Br2��CH2BrCH2Br ͭ˿�ɺ�ɫ���ɫ�������̼�����ζ 2CH3CH2OH+O2![]() 2CH3CHO+2H2O CH3COOH+HCO3-��CH3COO-+H2O+CO2�� B Ũ���� D Na2CO3 ��С����ˮ�е��ܽ�ȣ����ڷֲ㣩����ȥ��������������е����ᣬ�ܽ��������������е��Ҵ� ��������ˮ�� CH3COOH + HOCH2CH3
2CH3CHO+2H2O CH3COOH+HCO3-��CH3COO-+H2O+CO2�� B Ũ���� D Na2CO3 ��С����ˮ�е��ܽ�ȣ����ڷֲ㣩����ȥ��������������е����ᣬ�ܽ��������������е��Ҵ� ��������ˮ�� CH3COOH + HOCH2CH3 ![]() CH3COOCH2CH3 +H2O
CH3COOCH2CH3 +H2O
��������
�����л���ļ��ת����ϵ�����ʵ����ʷ�������Ӧ�����ɣ���д��ط�Ӧ����ʽ�������Ʊ����������ĵ�ʵ��ԭ������ʵ���в�����ע�����
A��һ����̬�����ڱ�״���µ��ܶ���1.25g/L����Ħ������=1.25g/L��22.4L/mol=28g/mol��������Ǻ���һ������ʯ�ͻ�����չˮƽ����AΪCH2=CH2��B��������C��C��������D��B��D���������г������л�����D�ķ���ʽ��֪��BΪCH3CH2OH��CΪCH3CHO��DΪCH3COOH���ʷ�Ӧ������ϩ��ˮ�����ӳɷ�Ӧ����CH3CH2OH��CH3CH2OH��CH3COOH��Ũ���������·���������Ӧ����������������FΪCH3COOCH2CH3����ϩ���巢���ӳɷ�Ӧ����EΪ1��2-�������飬
��1��������������֪��AΪ��ϩ���ṹʽΪ![]() ��BΪCH3CH2OH�����й�����Ϊ�ǻ����ǻ��ĵ���ʽΪ
��BΪCH3CH2OH�����й�����Ϊ�ǻ����ǻ��ĵ���ʽΪ![]() ��DΪCH3COOH�������Ȼ����ʴ�Ϊ��
��DΪCH3COOH�������Ȼ����ʴ�Ϊ��![]() ��
��![]() ���Ȼ���
���Ȼ���
��2����Ӧ������ϩ��ˮ�����ӳɷ�Ӧ�����Ҵ�����Ӧ������ϩ���巢���ӳɷ�Ӧ����1��2-�������飬��Ӧ����ʽΪ��CH2=CH2+Br2��CH2BrCH2Br���ʴ�Ϊ���ӳɷ�Ӧ��CH2=CH2+Br2��CH2BrCH2Br��
��3����Ӧ����Cu�������������½��У���ʵ��IJ����ǽ�������ͭ˿���ھƾ����ϼ��ȣ���ͭ˿��Ϊ��ɫʱ��Ѹ�ٽ�����뵽װ��B���Թ��У��ظ�����2-3�Σ��۲쵽�������ǣ�ͭ˿�ɺ�ɫ���ɫ�������̼�����ζ���÷�Ӧ�Ļ�ѧ����ʽΪ��2CH3CH2OH+O2![]() 2CH3CHO+2H2O��
2CH3CHO+2H2O��
��4��������̼��������Һ��Ӧ���������ơ�������̼��ˮ����Ӧ�����ӷ���ʽΪ��CH3COOH+HCO3-��CH3COO-+H2O+CO2����
��5���������Ҵ���Ũ���ᡢ�����������Ʊ�����������Ũ�����ܶȴ�Ϊ��ֹ����Σ�գ�������ʵ��ʱ�Թ�1��װҩƷ��˳��Ϊ�ȼ��Ҵ����ټ���Ũ���ᣬ�����������Ӧ��Ũ�������������ˮ�����ã��ñ��͵�̼������Һ�������������е��������Ҵ������������������ܽ�ȣ����ڷֲ㣬�÷�Ӧ����ʽΪ��CH3CH2OH+CH3COOH![]() CH3COOCH2CH3+H2O���ʴ�Ϊ�� B ��Ũ���D��Na2CO3����С����ˮ�е��ܽ�ȣ����ڷֲ㣩����ȥ��������������е����ᣬ�ܽ��������������е��Ҵ� ����������ˮ�� �� CH3CH2OH+CH3COOH
CH3COOCH2CH3+H2O���ʴ�Ϊ�� B ��Ũ���D��Na2CO3����С����ˮ�е��ܽ�ȣ����ڷֲ㣩����ȥ��������������е����ᣬ�ܽ��������������е��Ҵ� ����������ˮ�� �� CH3CH2OH+CH3COOH![]() CH3COOCH2CH3+H2O��
CH3COOCH2CH3+H2O��

 ȫ�ܲ����ĩС״Ԫϵ�д�
ȫ�ܲ����ĩС״Ԫϵ�д�����Ŀ��������������������⣺
(1)��ҵ������CO��ˮ������һ�������·�����Ӧ��ȡ������
CO(g)��H2O(g) ![]() CO2(g)��H2(g) ��H����41 kJ/mol
CO2(g)��H2(g) ��H����41 kJ/mol
��֪��2H2 (g) + O2 (g) = 2H2O (g) ��H����484 kJ/mol��д��CO��ȫȼ������CO2���Ȼ�ѧ����ʽ��_______________________________________��
(2)���Ŵ�����Ⱦ���������أ������ܼ�����������ȫ�����������ŷţ��о�NO x��SO2��CO�ȴ�����Ⱦ����Ĵ���������Ҫ���塣�û���̿��ԭ��������������йط�ӦΪ:C(s)��2NO(g)![]() N2(g)��CO2(g)��ij�о�С����ij�ܱ���������һ�����Ļ���̿��NO������(T1��)�����·�Ӧ����Ӧ���е���ͬʱ���ø����ʵ�Ũ�����£�
N2(g)��CO2(g)��ij�о�С����ij�ܱ���������һ�����Ļ���̿��NO������(T1��)�����·�Ӧ����Ӧ���е���ͬʱ���ø����ʵ�Ũ�����£�
Ũ��/mol��L��1 ʱ��/min | NO | N2 | CO2 |
0 | 0.100 | 0 | 0 |
10 | 0.058 | 0.021 | 0.021 |
20 | 0.040 | 0.030 | 0.030 |
30 | 0.040 | 0.030 | 0.030 |
40 | 0.032 | 0.034 | 0.017 |
50 | 0.032 | 0.034 | 0.017 |
����ӷ�Ӧ��ʼ��20miʱ����NO��ʾ��ƽ����Ӧ����= ________�����¶��¸÷�Ӧ��ƽ�ⳣ��K��____��������λС����
��30min�ı�ijһ��������Ӧ���´ﵽƽ�⣬��ı������������_____��дһ�����ɣ���
��������������˵��������Ӧ�Ѵ�ƽ�����__________��
A�������������ƽ��Ħ���������ֲ���
B��2v(NO)��=v(N2)��
C�������������ѹǿ���ֲ���
D����λʱ��������nmolCO2��ͬʱ����2nmolNO
(3)����Fe2+��Fe3+�Ĵ����ã������½�SO2ת��ΪSO42���� ��ʵ��SO2�Ĵ������ܷ�ӦΪ2SO2+O2+2H2O��2H2SO4������֪����SO2�ķ���ͨ�뺬Fe2+��Fe3+����Һʱ������һ����Ӧ�����ӷ���ʽΪ4Fe2+ + O2+ 4H+ ��4Fe3+ + 2H2O������һ��Ӧ�����ӷ���ʽΪ_______________
(4)��ѧ��������ͼ��ʾװ����ԭ���ԭ����SO2ת��Ϊ��Ҫ�Ļ���ԭ�ϡ�

��AΪSO2��BΪO2��CΪH2SO4������ӦʽΪ_______________________________������ܷ�ӦʽΪ________________________��
����Ŀ������Ҫ��ش��������⣺
(1)�ô���ʯ��100mlijϡ���ᷴӦ��CO2 ������CO2������뷴Ӧʱ��Ĺ�ϵ��ͼ��ʾ�����в���CO2��������Ϊ____��(ѡ����OE������EF������FG��)�����ö������ɱ�״���µ�CO2 448mL������ʱ��Ϊ2min,�ö�ʱ������HCl����ʾ�䷴Ӧ���ʣ�������Ϊ_____��(���Է�Ӧ����Һ����ı仯),��Ҫʹ�÷�Ӧ�ķ�Ӧ���ʼӿ죬���д�ʩ���е���______(����ĸ)��

A.������ʯ����̼��Ʒ�ĩ B.����K2SO4��Һ C.�����¶�
D.����NaCl���� E.���������Ũ��
(2)���ڷ�Ӧ2A2+B2=2A2B����֪A2��B2��A2B�ļ������±���
��ѧ�� | A-A | B=B | A-B |
����/kJ/mol | 236 | 406 | 163 |
��ͼ����ȷ��ʾ�÷�Ӧ�����������仯��ѡ����_______(����ĸ)��