��Ŀ����
17�������ƻ���к��е��ۡ������Ǻ����εȣ�ij������ȤС�������һ��ʵ��֤��ijЩ�ɷݵĴ��ڣ�������벢Э������������ʵ�飮��1����С�Թ�ȡ������ƻ��֭����������Cu��OH��2����Һ�������ȣ�����ש��ɫ�ij�������ƻ���к���C6H12O6��д����ʽ����
��2����������һ�������¿��Եõ���ѧʽΪC2H6O�Ļ�����A��A+CH3COOH������ζ�IJ����A���������Ϊ75%��ˮ��Һ����������������
��3��ƻ���к���ƻ���ᣬ�������Է�������Ϊ134��ȡ0.02molƻ���ᣬʹ����ȫȼ�գ���ȼ�պ�IJ����Ⱥ�ͨ����������ˮCaCl2�ͼ�ʯ�ң����߷ֱ�����1.08g �� 3.52g���������C��Hԭ�ӵĸ�����2��3��ƻ����ķ���ʽ��C4H6O5��
���� ��1����������Cu��OH��2����Һ�������ȣ�����ש��ɫ�ij���˵������ȩ����
��2��AΪ�Ҵ�����A���������Ϊ75%��ˮ��Һ������ɱ��������
��3����һ�������л���ȼ�ղ���ɼ����л����ʵ��ʽ���ٸ�����Է��������ɼ��㲢д������ʽ��
��� �⣺��1����������Cu��OH��2����Һ�������ȣ�����ש��ɫ�ij���˵������ȩ����ƻ���к��������ǣ�����ʽΪC6H12O6���ʴ�Ϊ��C6H12O6��
��2����������һ�������¿��Եõ���ѧʽΪC2H6O�Ļ�����A��A+CH3COOH������ζ�IJ��˵��AΪ�Ҵ������Ҵ����������Ϊ75%��ˮ��Һ������������
�ʴ�Ϊ����������
��3��ʹ��ˮCaCl2���ؿ�֪ˮ������Ϊ1.08g���ɼ����n��H2O��=$\frac{1.08g}{18g/mol}$=0.06 mol��n��H��=0.12 mol��ʹ��ʯ������3.52g����֪������̼����Ϊ3.52g��
n��C��=n��CO2��=$\frac{3.52g}{44g/mol}$=0.08 mol��1molƻ���Ậ��ԭ��n��H��=6 mol��n��C��=4 mol����n��O����$\frac{134g-6g-4��12g}{16g/mol}$=5mol��
������C��Hԭ�ӵĸ�����Ϊ0.08mol��0.12mol=2��3��
��n��C����n��H����n��O��=4mol��6mol��5mol=4��6��5������ʽΪC4H6O5��
�ʴ�Ϊ��2��3��C4H6O5��
���� ���⿼���л���Ľṹ�����ʡ�����ʽ��ȷ���ȣ���Ŀ��Ϊ�ۺϣ���һ�����Ѷȣ�����ʱע��������йؼ���Ϣ��ͬʱ����ѧ����������ͽ�������������

��ʯ��ˮ ��H2S��Һ ��KMnO4��Һ ����ˮ ���ữ��Ba��NO3��2��Һ ��Ʒ����Һ��
| A�� | ֻ�Т� | B�� | �٢� | C�� | �٢ڢۢ� | D�� | �ڢۢܢ� |
| A�� | �����£�7.8g����Na2O2�У����е�������������Ϊ0.4NA | |
| B�� | 4��ʱ��18g2H216O�к��й��õ��Ӷ���Ϊ2NA | |
| C�� | �ý�������CuƬ��ϡ�������ԭ��أ�����������������5.6gʱ���������·�ĵ���Ϊ0.3NA | |
| D�� | 1mol N5+���еĵ�����Ϊ34NA |
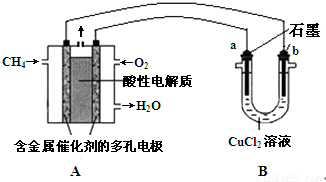
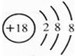 ��
��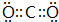 ��B��D�γɵ�ԭ�Ӹ�����Ϊ1��1�Ļ�����ĵ���ʽ��
��B��D�γɵ�ԭ�Ӹ�����Ϊ1��1�Ļ�����ĵ���ʽ��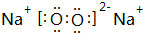 ��
��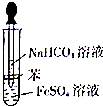 ijУ��ѧ��ȤС��Ϊ̽��FeSO4��NaHCO3�ķ�Ӧ������ͼ��ʾ������NaHCO3��Һ�μӵ�FeS04��Һ�У�FeS04��NaHCO3��Һ���þ���к���ȴ������ˮ���ƣ�����FeSO4��Һ�м����������ۣ����۲쵽�Թ����������ְ�ɫ������ͬʱ�д�����ɫ�������ɣ�
ijУ��ѧ��ȤС��Ϊ̽��FeSO4��NaHCO3�ķ�Ӧ������ͼ��ʾ������NaHCO3��Һ�μӵ�FeS04��Һ�У�FeS04��NaHCO3��Һ���þ���к���ȴ������ˮ���ƣ�����FeSO4��Һ�м����������ۣ����۲쵽�Թ����������ְ�ɫ������ͬʱ�д�����ɫ�������ɣ�