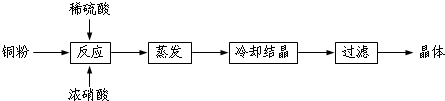
��Ŀ����
9���������м������ʣ���CaS ��Ar ��CaO2 �ܽ��ʯ ��SiC ��H2O2 �ߣ�NH4��2SO4��MgCl2 ��CH3COONa ��[Cu��NH3��4]SO4 �����¿ո����д��ţ�
��1���������Լ��ķ��Ӿ����Ǣڣ�
��2�����м��Լ��ķ��Ӿ����Ǣޣ�
��3��ֻ�����Ӽ������Ӿ����Ǣ٢ࣻ
��4�����м��Լ���ԭ�Ӿ����Ǣݣ�
��5�����зǼ��Լ������Ӿ����Ǣۢ
��6��������λ�������Ӿ����Ǣߢ⣮
���� һ����˵�����ý����ͻ��÷ǽ���Ԫ��֮�����γ����Ӽ����ǽ���Ԫ��֮�����γɹ��ۼ���ֻ�����ۼ��Ļ�����Ϊ���ۻ�������ۻ�������һ���������Ӽ����������ӵĻ�����Ϊ���ӻ�������ӻ������п��ܺ��й��ۼ������Ӿ��������Ӽ��������Ϊ���Ӽ������������Ӿ��������Ӽ��������Ϊ���Ӽ���ԭ�Ӿ�����ԭ�Ӽ��������Ϊ���ۼ���
��� �⣺��CaS�и����Ӻ�������֮��������Ӽ���Ϊ���Ӿ��壻
��Ar��û�л�ѧ�������ڷ��Ӿ��壻
��CaO2�и����Ӻ���������֮��������Ӽ���Oԭ��֮����ڷǼ��Լ���Ϊ���Ӿ��壻
�ܽ��ʯ�к��зǽ����Լ�������ԭ�Ӿ��壻
��SiC�к��м��Լ�������ԭ�Ӿ��壻
��H2O2 �к��м��Լ��ͷǼ��Լ������ڷ��Ӿ��壻
�ߣ�NH4��2SO4�к��м��Լ������Ӽ�����λ�����������Ӿ��壻
��MgCl2��þ���Ӻ�������֮��������Ӽ���Ϊ���Ӿ��壻
��CH3COONa�д��ڼ��Լ����Ǽ��Լ������Ӽ����������Ӿ��壻
��[Cu��NH3��4]SO4 �д��ڼ��Լ�����λ�������Ӽ����������Ӿ��壻
��1���������Լ��ķ��Ӿ����� �ڣ��ʴ�Ϊ���ڣ�
��2�����м��Լ��ķ��Ӿ����� �ޣ��ʴ�Ϊ���ޣ�
��3��ֻ�����Ӽ������Ӿ����� �٢ࣻ�ʴ�Ϊ���٢ࣻ
��4�����м��Լ���ԭ�Ӿ����� �ݣ��ʴ�Ϊ���ݣ�
��5�����зǼ��Լ������Ӿ����� �ۢ�ʴ�Ϊ���ۢ
��6��������λ�������Ӿ����� �ߢ⣻�ʴ�Ϊ���ߢ⣮
���� ���⿼���˻�ѧ���ͻ�����Ĺ�ϵ�����ݻ�����������д��ڵĻ�ѧ�����������ע�ⲻ�ܸ����Ƿ��н���Ԫ��ȷ�����Ӽ���������в�������Ԫ�أ����������Ӽ�����Ŀ�ѶȲ���

| A�� | ��ʳ�׳�ȥůˮƿ�е�ˮ�� | |
| B�� | ���ȵĴ�����Һϴ�Ӳ;��ϵ��� | |
| C�� | ����������ʳ�����Ƿ���� | |
| D�� | �����ղ�����ζ�ķ��������ʹ���ë֯�� |
| A�� | 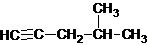 2-��-4-��Ȳ 2-��-4-��Ȳ | |
| B�� | CH2=C��C2H5��22-�һ�-1-��ϩ | |
| C�� | CH3CH��CH3��CH2CH��C2H5��CH3 2-��-4-�һ����� | |
| D�� | 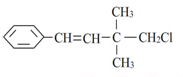 1��3��5-�������ױ� 1��3��5-�������ױ� |
| A�� | ��̬ԭ���Ǵ����������״̬��ԭ�� | |
| B�� | ��̬Cԭ�ӵĵ����Ų�ʽ��1s22s12p3 | |
| C�� | ��ɫ��Ӧ�ǽ���ԭ�ӵĵ��Ӵӻ�̬ԾǨ������̬ʱ�����Ĺ��� | |
| D�� | ͬһԭ�Ӵ��ڼ���̬ʱ������һ�����ڻ�̬ʱ������ |
��1����Ļ�̬ԭ�ӵļ۵����Ų�ͼΪ4s24p5��
��2���ڲ�̫ϡ����Һ�У���������Զ����ӵϣ�HF��2��ʽ���ڵģ�ʹ�������ӵϵ��������������
��3��������±��ṩ�ĵ�һ�����������жϣ����п������ɽ��ȶ��ĵ��������ӵ�±��ԭ���ǵ⣨I����
| Ԫ������ | �� | �� | �� | �� |
| ��һ�����ܣ�kJ/mol�� | 1681 | 1251 | 1140 | 1008 |
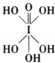 ����HIO4��ǰ��Ϊ��Ԫ�ᣬ����ΪһԪ�ᣮ��Ƚ϶�������ǿ����H5IO6��HIO4�������������������=����
����HIO4��ǰ��Ϊ��Ԫ�ᣬ����ΪһԪ�ᣮ��Ƚ϶�������ǿ����H5IO6��HIO4�������������������=������5������ˮ�е��ܽ����ȻС�����ڵ⻯����Һ���ܽ��ȴ������������������Һ�з������з�Ӧ��I-+I2�TI3-��I3-������ԭ����Χ�ĦҼ����ӶԶ���Ϊ2���µ��ӶԶ���Ϊ3��
��6��д��һ��CO2�ĵȵ����壺CS2��N2O��
| A�� | �϶����ڢ� | B�� | ���ٴ��ڢں͢� | C�� | ��ȷ���Ƿ��Т� | D�� | ���ٴ��ڢ١��ܡ��� |
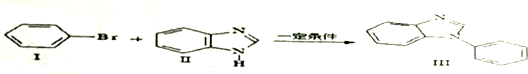
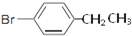 ��
��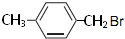 ��
��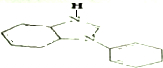 ��
��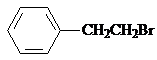 �������������ƴ���Һ���������·�Ӧ�ķ���ʽΪ��
�������������ƴ���Һ���������·�Ӧ�ķ���ʽΪ��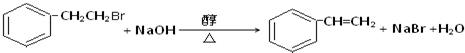 ��
��