��Ŀ����
����Ŀ����þ�������ס����Ԫ�صĻ������ڻ���������ҩ�ﻯѧ������ѧ������Ӧ�ù㷺���ش���������:
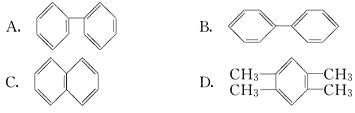
(1)����״̬��þ�У����������һ��������������������_____������ţ���
A��![]() B��
B��![]() C��
C��![]() D��
D��![]()
(2)�������ֽе����ܡ�����1mol ���ӻ������е����������Ӵ���������̬��ϳ����Ӿ���ʱ���ų���������MgO �����ܿ�ͨ����ͼ��ʾ��Borm Haberѭ������õ���
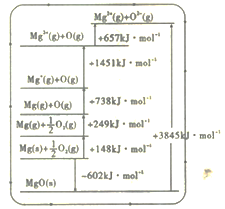
Mg�ĵڶ�������Ϊ______kJ��mol-1��MgO�ľ�����Ϊ___________kJ��mol-1��
(3)�Ҷ�����H2NCH2CH2NH2�� ��һ���л����������Nԭ�ӵ��ӻ�������____�����л�����������Mg2+��Cu2+�Ƚ��������γ��ȶ���״���ӣ���ԭ����______���������Ҷ����γɵĻ������ȶ�����Խϸߵ���_______���� ��Mg2+ ������Cu2+�� ����
(4) PCl5��һ�ְ�ɫ���壬���ȵ�160��C������Һ̬�ξͱ�����������180��C�µ������ܶȣ��ۺϳɱ�״����Ϊ9.3g��L-1�����ӵļ���Ϊ�㣬P-Cl����Ϊ204pm��211pm���֡���180��C��PCl5�����д��ڵķ�����ʽΪ____���ѧʽ�������ӵĿռ乹��Ϊ______��P��Cl�ĵ縺���ɴ�С˳��Ϊ________��
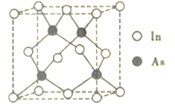
(5)�������ɵ�һ�ֻ��������ڰ뵼����ϣ��侧���ṹ��ͼ��ʾ����֪�����IJ���Ϊa pm�������ӵ�������ֵΪNA����þ�����ܶ�Ϊ_____g. cm-3 ���ú�a��NA�Ĵ���ʽ��ʾ����
���𰸡�C 1451 3845 sp3 �Ҷ���������Nԭ���ṩ�¶Ե��Ӹ����������γ���λ�� Cu2+ PCl5 ����˫�� Cl>P ![]()
��������
(1)A��![]() �����1�����ӣ�����Mg�ĵ�һ�����ܣ�
�����1�����ӣ�����Mg�ĵ�һ�����ܣ�
B��![]() �����1�����ӣ�����Mg�ĵ�һ�����ܣ�
�����1�����ӣ�����Mg�ĵ�һ�����ܣ�
C��![]() �����1�����ӣ�����Mg�ĵڶ������ܣ���s���ӵ������ͣ�
�����1�����ӣ�����Mg�ĵڶ������ܣ���s���ӵ������ͣ�
D��![]() �����1�����ӣ�����Mg�ĵڶ������ܣ���p��������ϸߣ�
�����1�����ӣ�����Mg�ĵڶ������ܣ���p��������ϸߣ�
(2)Mg�ĵڶ��������ǽ�Mg+ת��ΪMg2+ʱ���յ�������MgO�ľ�����Ϊ��Mg2+(g)��O2-(g)�������MgO(s)���ͷŵ�������
(3)�Ҷ�����H2NCH2CH2NH2����һ���л����������Nԭ�ӵļ۲���Ӷ���Ϊ4 ���ɴ˵ó��ӻ����ͣ����л�����������Mg2+��Cu2+�Ƚ��������γ��ȶ���״���ӣ��ɴ��γ���λ������������ԭ�����Ҷ����γɵĻ������ȶ�����Խϸߵ����ӣ��ɴ����Ӱ뾶���չ�����з�����
(4)����M=��Vm���м��㣬�����180��CʱPCl5�����д��ڵķ�����ʽ�����ԭ���γɵķǼ��Է��ӣ����ӵĿռ乹��Ϊ����˫��P��Cl�ĵ縺����ǽ����Գ����ȡ�
(5)���������������ԭ���������1������������������������øþ�����ܶȡ�
(1)A��![]() �����1�����ӣ�����Mg�ĵ�һ�����ܣ�
�����1�����ӣ�����Mg�ĵ�һ�����ܣ�
B��![]() �����1�����ӣ�����Mg�ĵ�һ�����ܣ�
�����1�����ӣ�����Mg�ĵ�һ�����ܣ�
C��![]() �����1�����ӣ�����Mg�ĵڶ������ܣ���s���ӵ������ͣ����ܶࣻ
�����1�����ӣ�����Mg�ĵڶ������ܣ���s���ӵ������ͣ����ܶࣻ
D��![]() �����1�����ӣ�����Mg�ĵڶ������ܣ���p��������ϸߣ����������C��ҪС��
�����1�����ӣ�����Mg�ĵڶ������ܣ���p��������ϸߣ����������C��ҪС��
��ѡC����Ϊ��C��
(2)Mg�ĵڶ��������ǽ�Mg+ת��ΪMg2+ʱ���յ��������ӱ��п���ȡ������Ϊ1451 kJ��mol-1��MgO�ľ�����Ϊ��Mg2+(g)��O2-(g)�������MgO(s)���ͷŵ��������ӱ��п���ȡ������Ϊ3845 kJ��mol-1����Ϊ��1451��3845��
(3)�Ҷ�����H2NCH2CH2NH2����һ���л����������Nԭ�ӵļ۲���Ӷ���Ϊ4 ���ɴ˵ó��ӻ�����Ϊsp3�����л�����������Mg2+��Cu2+�Ƚ��������γ��ȶ���״���ӣ���ԭ�����Ҷ���������Nԭ���ṩ�¶Ե��Ӹ����������γ���λ�������Ҷ����γɵĻ������ȶ�����Խϸߵ�����Cu2+����ΪCu2+�İ뾶�ϴ��Һ��еĿչ������Mg2+����Ϊ��sp3���Ҷ���������Nԭ���ṩ�¶Ե��Ӹ����������γ���λ����
(4)M=��Vm=9.3g��L-1��22.4L/mol=208g/mol�������180��CʱPCl5�����д��ڵķ�����ʽΪPCl5�����ԭ���γɷǼ��Է��ӣ����ӵĿռ乹��Ϊ����˫����Ϊ�ǽ�����Cl>P ������P��Cl�ĵ縺��Cl>P����Ϊ��PCl5������˫�ͣ�Cl>P��
(5)������������Inԭ����Ϊ8��![]() +6��
+6��![]() =4������Asԭ����Ϊ4����þ�����ܶ�Ϊ
=4������Asԭ����Ϊ4����þ�����ܶ�Ϊ![]() =
=![]() ������
������![]() ��
��

����Ŀ������������ʵ�����������õ���Ⱥ͵���ƽ�ⳣ����ʾ���±��dz����¼�������ĵ���ƽ�ⳣ��(Ka)������ĵ���ƽ�ⳣ��(Kb)
���� | ����ƽ�ⳣ��(Ka��Kb) |
CH3COOH | 1.8��10-5 |
HNO2 | 4.6��10-4 |
HCN | 5��10-10 |
HClO | 3��10-8 |
NH3H2O | 1.8��10-5 |
�±��dz����¼�����()������ܶȻ�����(Ksp)��
��()���� | �ܶȻ�����(Ksp) |
BaSO4 | 1��10-10 |
BaCO3 | 2.6��10-9 |
CaSO4 | 7��10-5 |
CaCO3 | 5��10-9 |
��ش��������⣺
(l)д��HCN�ĵ��뷽��ʽ��___________��HClO�ĵ���ʽ__________��
(2)�����������������У�������ǿ����__________(�û�ѧʽ��ʾ)��������ʹ������Һ��CH3COOH�ĵ���̶������ҵ��볣���ı�IJ�����__________(����ĸ���)��
A.������������ B.�����¶� C.��ˮϡ�� D.�����¶� E.��������CH3COONa����
(3)CH3COONH4��ˮ��Һ��__________(ѡ������������������������������)������Һ�д��ڵĸ�����Ũ�ȴ�С��ϵ��__________��
(4)��ҵ�г���BaSO4ת��ΪBaCO3���ٽ����Ƴɸ��ֿ����Եı���(��BaCl2)�������������ñ��͵Ĵ�����Һ����BaSO4��ĩ�������ϲ��䴿����BaSO4ת��ΪBaCO3����������BasO4����Һ���ڸ�����Һ�мӴ����ĩ�����Ͻ��裬ΪʹSO42�����ʵ���Ũ�Ȳ�С��0.02 mol��L��1������Һ��CO32�����ʵ���Ũ��Ӧ��__________mol��L��1��