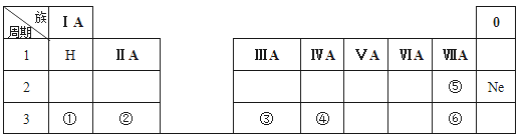
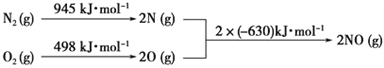
��Ŀ����
����Ŀ��Ϊ�˲ⶨij�л���A�Ľṹ��������ʵ�飺
�ٽ�2.3 g���л�����ȫȼ�գ�����0.1 mol CO2��2.7 gˮ��
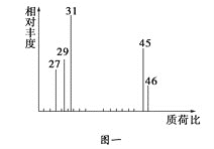
���������Dzⶨ����Է�������������ͼһ��ʾ������ͼ��
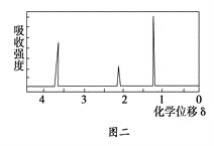
���ú˴Ź����Ǵ����û�����õ���ͼ����ʾͼ�ף�ͼ������������֮����1��2��3���Իش��������⣺
��1���л���A����Է���������________��
��2���л���A��ʵ��ʽ��________��
��3��A�ķ���ʽ��_______________________________________��
��4��A�Ľṹ��ʽΪ___________________________________________��
���𰸡�46 C2H6O C2H6O CH3CH2OH
��������
��1�������ʺɱ��ж��л���A����Է���������
��2������n=m��M�������ɵ�ˮ�����ʵ���������̼Ԫ�ء���Ԫ�ص����������������غ��ж��л���A�Ƿ�����Ԫ�أ���������Ԫ�أ�������Ԫ����������ԭ�����ʵ����������л���A��C��H��Oԭ�Ӹ�����ֵȷ��ʵ��ʽ��
��3�������л����ʵ��ʽ��Hԭ��˵���Ƿ�Cԭ�ӵ��ļ۽ṹ�жϣ�
��4���ɺ˴Ź��������жϸ��л��ﺬ��3��H����ȷ�����ӵĽṹ��ʽ��
��1����A������ͼ�У�����ʺɱ�Ϊ46����������Է�������Ҳ��46��
��2��2.3 g���л�����n��C��=n��CO2��=0.1 mol�����е�̼ԭ�ӵ�����Ϊm��C��=0.1 mol��12 gmol-1=1.2 g����ԭ�ӵ����ʵ���Ϊ��n��H��=2��2.7g/18 gmol-1=0.3 mol����ԭ�ӵ�����Ϊm��H��=0.3 mol��1 gmol-1=0.3 g�����л�����m��O��=2.3g-1.2 g-0.3g=0.8g����Ԫ�ص����ʵ���Ϊn��O��=0.8 g��16g/mol=0.05 mol����n��C����n��H����n��O��=0.1 mol��0.3 mol��0.05 mol=2��6��1������A��ʵ��ʽ��C2H6O��
��3�����л�������ʽΪC2H6O��Hԭ���Ѿ�����Cԭ�ӵ��ļ۽ṹ�����ʽ��Ϊ����ʽ��
��4��A���������ֿ��ܵĽṹ��CH3OCH3��CH3CH2OH����Ϊǰ�ߣ����ں˴Ź���������Ӧֻ��1���壻��Ϊ���ߣ����ں˴Ź���������Ӧ��3���壬����3��������֮����1��2��3����ȻCH3CH2OH�������⣬����AΪ�Ҵ����ṹ��ʽΪCH3CH2OH��