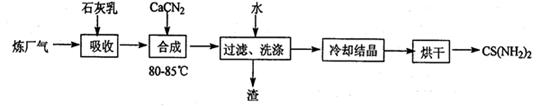
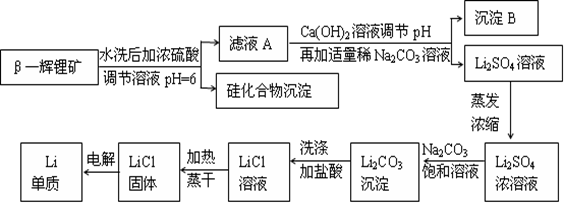
��Ŀ����
��ʯ���顢ʯ�ҵ�(CaCN2)������������H2S����Ӧ�����ܾ���β�������ܻ��Ӧ�ù㷺��CS(NH2)2(����)���䲿�ֹ����������£�
��1�������£�H2S�������з�Ӧ:2H2S(g) 2H2(g)+S2(g),��ƽ�ⳣ������ʽΪK=��
2H2(g)+S2(g),��ƽ�ⳣ������ʽΪK=��
��2����ʯ��������H2S��ȡCa(HS)2��Ҫ�ڵ����½��У���ԭ������ �����˵õ��������������ã���������Ҫ�ɷ����� (�ѧʽ)��
��3���ϳ������賤ʱ����裬���ڽϸ��¶�(80��-85��)�½��У���Ŀ������ ��
Ca(HS)2��CaCN2��ˮ��Һ�кϳ������Ļ�ѧ����ʽΪ�� ��
��4��������X�����廥Ϊͬ���칹�壬X����FeCl3��Һ�У���Һ�Ժ�ɫ��X�Ļ�ѧʽΪ�� ��

��1�������£�H2S�������з�Ӧ:2H2S(g)
 2H2(g)+S2(g),��ƽ�ⳣ������ʽΪK=��
2H2(g)+S2(g),��ƽ�ⳣ������ʽΪK=����2����ʯ��������H2S��ȡCa(HS)2��Ҫ�ڵ����½��У���ԭ������ �����˵õ��������������ã���������Ҫ�ɷ����� (�ѧʽ)��
��3���ϳ������賤ʱ����裬���ڽϸ��¶�(80��-85��)�½��У���Ŀ������ ��
Ca(HS)2��CaCN2��ˮ��Һ�кϳ������Ļ�ѧ����ʽΪ�� ��
��4��������X�����廥Ϊͬ���칹�壬X����FeCl3��Һ�У���Һ�Ժ�ɫ��X�Ļ�ѧʽΪ�� ��
��1��c2(H2)��c(S2)/c2(H2S)
��2���¶ȵ�H2S�ܽ�������Һ��H2SŨ�ȸߣ����������գ�Ca(OH)2[��Ca(OH)2��CaS]
��3��ʹ��Ӧ���ֽӴ���ά�ֽϸ��¶ȣ���������߷�Ӧ����
Ca(HS)2+2CaCN2+6H2O
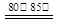 2CS(NH2)2 + 3Ca(OH)2
2CS(NH2)2 + 3Ca(OH)2��4��NH4SCN
�����������1��K�ı���ʽΪ������Ũ�ȵĴη����Է�Ӧ��Ũ�ȵĴη�����2��������ˮ�е��ܽ�ȣ������¶ȵ����߶���С��ʯ������Ca(OH)2��Ũ�Ƚϴ�����H2S��һ������ʣ�࣬���������á���3���Ӵ�������¶ȸ߾�������߷�Ӧ���ʡ��ɣ�2������ʾ֪��ʯ��������H2S����Ca(HS)2���롰�ϳɡ��Σ���ͷָ��Ϊ���뷴Ӧ���CaCN2��H2O��Ϊ��Ӧ���Ԫ���غ�֪������CS(NH2)2���Ca(OH)2����4����FeCl3��Һ����Һ�Ժ�ɫ��ӦΪSCN����ͬ���칹��Ļ�ѧʽ��ͬ����ԭ���������Ŀ����ȷ������NH4�� ����ΪNH4SCN��

��ϰ��ϵ�д�
�����Ŀ
 ,��Ӧ�¶����������档
,��Ӧ�¶����������档 
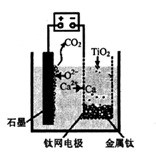
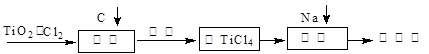
 TiCl4(g)+2CO (g) ���Լ����䷴Ӧ�ġ�H= kJ?mol��1����Ӧ��ƽ�ⳣ������ʽK= ������ͼ������TiCl4�ﵽƽ���ٷֺ������¶ȵı仯����ͼ��
TiCl4(g)+2CO (g) ���Լ����䷴Ӧ�ġ�H= kJ?mol��1����Ӧ��ƽ�ⳣ������ʽK= ������ͼ������TiCl4�ﵽƽ���ٷֺ������¶ȵı仯����ͼ��

 Al(OH)3+3H+��ƽ�ⳣ��Ϊ ��������λ��Ч���֣���
Al(OH)3+3H+��ƽ�ⳣ��Ϊ ��������λ��Ч���֣���