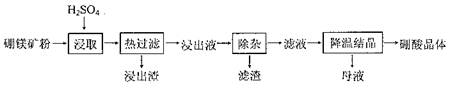
��Ŀ����
�������ԭ���ܹ�ҵ������Դ��ҵ�е���Ҫ���ʡ���ҵ�ϳ��æ¡���﮿�(��Ҫ�ɷ���LiAlSi2O6�����������ơ�þ����)�Ʊ�����ﮣ��������������£�
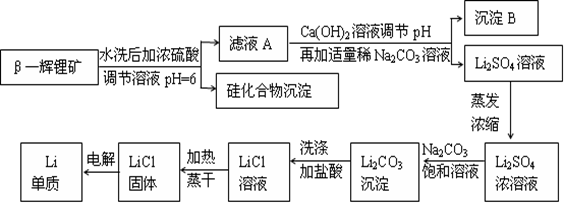
��֪Li2CO3����ˮ����ش��������⣺
��1��д��LiAlSi2O6�����ᷴӦ�Ļ�ѧ����ʽ_______________________��
��2������B����Ҫ�ɷ���_____________(д��ѧʽ)��
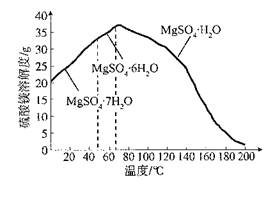
��3������Ũ��Li2SO4��Һʱ����Ҫʹ�õĹ�������������������_________��_________��
��4��������������������Ũ��Li2SO4��Һ��Ŀ����____________________��
��5�����������﮵��ʱ������FeF3���������Ļ������ʣ�FeF3����FeCl3��40%HF��Һ��Ӧ�Ʊ������Ʊ���������Ҫѡ�����ƾ��ķ���ϩ���ϵ��������з�Ӧ������������ͨ�IJ����������մ���������ԭ����_________________________________(�û�ѧ��Ӧ����ʽ��ʾ)��
��6������﮿����ڴ�����������ԭ���ǣ���2Li��H2��2LiH����LiH��H2O��LiOH��H2��������֪LiH���ܶ�Ϊ0.82g��cm-3���ý��������224L H2����״����ǡ����ȫ��Ӧ�������ɵ�LiH������뱻���յ����������֮��Ϊ1: ______(��ȷ������)��

��֪Li2CO3����ˮ����ش��������⣺
��1��д��LiAlSi2O6�����ᷴӦ�Ļ�ѧ����ʽ_______________________��
��2������B����Ҫ�ɷ���_____________(д��ѧʽ)��
��3������Ũ��Li2SO4��Һʱ����Ҫʹ�õĹ�������������������_________��_________��
��4��������������������Ũ��Li2SO4��Һ��Ŀ����____________________��
��5�����������﮵��ʱ������FeF3���������Ļ������ʣ�FeF3����FeCl3��40%HF��Һ��Ӧ�Ʊ������Ʊ���������Ҫѡ�����ƾ��ķ���ϩ���ϵ��������з�Ӧ������������ͨ�IJ����������մ���������ԭ����_________________________________(�û�ѧ��Ӧ����ʽ��ʾ)��
��6������﮿����ڴ�����������ԭ���ǣ���2Li��H2��2LiH����LiH��H2O��LiOH��H2��������֪LiH���ܶ�Ϊ0.82g��cm-3���ý��������224L H2����״����ǡ����ȫ��Ӧ�������ɵ�LiH������뱻���յ����������֮��Ϊ1: ______(��ȷ������)��
��1�� 2LiAlSi2O6��4H2SO4��Li2SO4��Al2(SO4) 3��4H2SiO3��(2��)��
(2) Al(OH)3��CaCO3 (2��) (3)�ƾ���(1��) ������(1��)��
(4)̼�������ˮ������Ũ����Ŀ����������Һ�������Ũ�ȣ�ʹ�����Ũ����̼�������Ũ��֮������Ksp(Li2CO3)���Ա����̼��﮳�����(3��)
(5)SiO2��4HF��SiF4����2H2O(2��)��(6) 1148(2��)��
(2) Al(OH)3��CaCO3 (2��) (3)�ƾ���(1��) ������(1��)��
(4)̼�������ˮ������Ũ����Ŀ����������Һ�������Ũ�ȣ�ʹ�����Ũ����̼�������Ũ��֮������Ksp(Li2CO3)���Ա����̼��﮳�����(3��)
(5)SiO2��4HF��SiF4����2H2O(2��)��(6) 1148(2��)��
����������¡���﮿�����������������ﮡ���������������������˳�ȥ���ᣬ��Һ�м���Ca(OH)2��Na2CO3������CaCO3��Al(OH)3���dz���B�����ˣ��õ��������Һ������Ũ������Na2CO3�õ�Li2CO3������������õ�LiCl������ﮡ���6����������10Ħ��������LiH20Ħ����������160�ˣ������
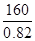 �������������22400�����������Ϊ1:1148
�������������22400�����������Ϊ1:1148
��ϰ��ϵ�д�
 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
�����Ŀ

 2H2(g)+S2(g),��ƽ�ⳣ������ʽΪK=��
2H2(g)+S2(g),��ƽ�ⳣ������ʽΪK=��