��Ŀ����
��14�֣� ������Ϊһ������Դ�ڻ�ѧ����Ӧ�ù㷺����ش��������⣺?
��1������Ȼ��Ϊԭ����H2�Ǻϳɰ���һ����Ҫ��·�ߡ�����IJ��������ɵõ��ϳɰ���ԭ����H2���䷴Ӧʽ���£�
��CH ��g��+1/2O
��g��+1/2O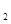 ��g����CO��g��+2H
��g����CO��g��+2H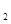 ��g��
��g��  H1����35.6kJ��mol
H1����35.6kJ��mol
���жϳ�����,������Ӧ�ܷ��Է�����: (��ܡ���)�����о���Ϊ���鲿�������Ļ���Ϊ��
��CH ��g��+2O
��g��+2O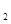 ��g����CO2��g��+2H2O��g��
��g����CO2��g��+2H2O��g��  H2����890.3kJ��mol
H2����890.3kJ��mol
��CH ��g��+CO
��g��+CO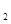 ��g����2CO��g��+2H
��g����2CO��g��+2H ��g��
��g��  H3��247.3kJ��mol
H3��247.3kJ��mol
������������д��CH4��H2O��g������CO��H2���Ȼ�ѧ��Ӧ����ʽ��
��
�ƺ����£���һ��2L���ܱ������г���1 molN2��2.6 molH2����Ӧ�����ж�NH3��Ũ�Ƚ��м�⣬�õ����������±���ʾ��
ʵ������
��������,�÷�Ӧ�ﵽ��ѧƽ��ʱ,������Ũ��Ϊ ��
��3������ͼ��ʾ��װ�â�Ϊ����ȼ�ϵ�أ��������ҺΪKOH��Һ����ͨ��װ�â�ʵ�������϶�ͭ��?
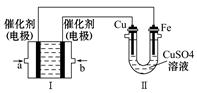
��b���缫�Ϸ����ĵ缫��Ӧʽ�� ��
�ڵ�ƽ�����װ�â�����Һ��pH ����д���������С�����䡱����ͬ����װ�â���Cu �����ʵ���Ũ�� ��
�����ʵ���Ũ�� ��
������ȫ��Ӧ��װ�â���������������12.8g����װ�â������������ļ��� L ����״���£���
��1������Ȼ��Ϊԭ����H2�Ǻϳɰ���һ����Ҫ��·�ߡ�����IJ��������ɵõ��ϳɰ���ԭ����H2���䷴Ӧʽ���£�
��CH
 ��g��+1/2O
��g��+1/2O ��g����CO��g��+2H
��g����CO��g��+2H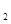 ��g��
��g��  H1����35.6kJ��mol
H1����35.6kJ��mol
���жϳ�����,������Ӧ�ܷ��Է�����: (��ܡ���)�����о���Ϊ���鲿�������Ļ���Ϊ��
��CH
 ��g��+2O
��g��+2O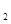 ��g����CO2��g��+2H2O��g��
��g����CO2��g��+2H2O��g��  H2����890.3kJ��mol
H2����890.3kJ��mol
��CH
 ��g��+CO
��g��+CO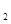 ��g����2CO��g��+2H
��g����2CO��g��+2H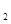 ��g��
��g��  H3��247.3kJ��mol
H3��247.3kJ��mol
������������д��CH4��H2O��g������CO��H2���Ȼ�ѧ��Ӧ����ʽ��
��
�ƺ����£���һ��2L���ܱ������г���1 molN2��2.6 molH2����Ӧ�����ж�NH3��Ũ�Ƚ��м�⣬�õ����������±���ʾ��
ʵ������
| ʱ��/min | 5 | 10 | 15 | 20 | 25 | 30 |
| c(NH3)/( mol ��/L-1) | 0.08 | 0.14 | 0.18 | 0.20 | 0.20 | 0.20 |
��3������ͼ��ʾ��װ�â�Ϊ����ȼ�ϵ�أ��������ҺΪKOH��Һ����ͨ��װ�â�ʵ�������϶�ͭ��?

��b���缫�Ϸ����ĵ缫��Ӧʽ�� ��
�ڵ�ƽ�����װ�â�����Һ��pH ����д���������С�����䡱����ͬ����װ�â���Cu
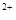 �����ʵ���Ũ�� ��
�����ʵ���Ũ�� ��������ȫ��Ӧ��װ�â���������������12.8g����װ�â������������ļ��� L ����״���£���
��14�֣���1���ܣ�1�֣�
CH ��g��+H2O��g����CO��g��+3H2��g��
��g��+H2O��g����CO��g��+3H2��g�� H��250.3kJ��mol
H��250.3kJ��mol ��3�֣�����ÿ��2��
��3�֣�����ÿ��2��
��2��0.4 mol ��L-1
��3���� O2��2H2O��4e��=4OH- �ڱ�С ���� �� 1.12
CH
 ��g��+H2O��g����CO��g��+3H2��g��
��g��+H2O��g����CO��g��+3H2��g�� H��250.3kJ��mol
H��250.3kJ��mol ��3�֣�����ÿ��2��
��3�֣�����ÿ��2����2��0.4 mol ��L-1
��3���� O2��2H2O��4e��=4OH- �ڱ�С ���� �� 1.12
��1���÷�ӦΪһ�������ķ��ȷ�Ӧ�������Է����У�ͨ����˹���ɣ�����д��Ŀ�귴ӦCH4(g)+H2O(g)=CO(g)+3H2(g)���÷�Ӧ����(�١�4-��-��)���ʦ�H=((-35.6)��4-(-890.3)-247.3)=250.3KJ/mol��
��2��
��3��������ͭ������ȷ��CuΪ������FeΪ������������ƹ����е����Ũ��û�б仯��
���Ϊȼ�ϵ�أ�aΪ����������ʧ���ӣ�bΪ������������ԭ��ӦO2��2H2O��4e��=4OH-������������KOH�������䣬����ˮ���ɣ�Ũ�ȼ�С��pH��С�����ݵ�ʧ�����غ����ȷ�������ļ���Ϊ1.12L
��2��
| | N2+3H2 2NH3 2NH3 | ||
| ʼ̬mol/L | 0.5 | 1.3 | 0 |
| ��Ӧmol/L | 0.1 | 0.3 | 0.20 |
| ��̬mol/L | 0.4 | 1.0 | 0.20 |
��3��������ͭ������ȷ��CuΪ������FeΪ������������ƹ����е����Ũ��û�б仯��
���Ϊȼ�ϵ�أ�aΪ����������ʧ���ӣ�bΪ������������ԭ��ӦO2��2H2O��4e��=4OH-������������KOH�������䣬����ˮ���ɣ�Ũ�ȼ�С��pH��С�����ݵ�ʧ�����غ����ȷ�������ļ���Ϊ1.12L

��ϰ��ϵ�д�
�����Ŀ
 2NO(g) DH��a kJ��mol��1��ƽ�ⳣ��K���±���
2NO(g) DH��a kJ��mol��1��ƽ�ⳣ��K���±��� C(g)+D(g)�����е������仯����ͼ��ʾ���жϸ÷�Ӧ��H 0�����������������
C(g)+D(g)�����е������仯����ͼ��ʾ���жϸ÷�Ӧ��H 0�����������������

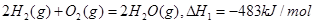 ��������Ȼ�ѧ����ʽ��
��������Ȼ�ѧ����ʽ��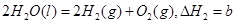 ������˵����ȷ���ǣ� ��
������˵����ȷ���ǣ� ��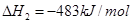
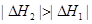
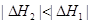
 2HBr(g) ��H=��72kJ/mol������1mol Br2(1)��Ҫ���յ�����Ϊ30kJ����������������±���
2HBr(g) ��H=��72kJ/mol������1mol Br2(1)��Ҫ���յ�����Ϊ30kJ����������������±��� l����ȫȼ�������ȶ�������ų�������ΪY kJ����1molP��O2��Ӧ���ɹ�̬P2O3�ķ�Ӧ�ȡ�H=________________________________��
l����ȫȼ�������ȶ�������ų�������ΪY kJ����1molP��O2��Ӧ���ɹ�̬P2O3�ķ�Ӧ�ȡ�H=________________________________��