��Ŀ����
��10�֣��ش��������⣺
��1����ӦA(g)+B(g) C(g)+D(g)�����е������仯����ͼ��ʾ���жϸ÷�Ӧ��H 0�����������������
C(g)+D(g)�����е������仯����ͼ��ʾ���жϸ÷�Ӧ��H 0�����������������
����ȷ��������

��2����Al2O3��Ni������̬���ᷢ�����з�Ӧ��
����(g)=" CO" (g)+ H2O (g)�� ��H1=" +34.0" kJ/mol
����(g)= CO2 (g)+ H2(g) ��H2=" ��7.0" kJ/mol
�����ķ���ʽΪ ���ڸ������£���̬CO2����̬H2��Ӧ������̬CO����̬H2O���Ȼ�ѧ����ʽΪ ��
��3����ͼ��ʾ��ˮ�����Թ�����һö��������������۲죺

I���Թ���Һ����������������Ӧ�� ��
II���Թ���Һ���½������� ��ʴ��
III����Һ��Ϊˮ����Һ��Ϊ��ˮ���������� ����ס����ҡ�����Һ�и�ʴ���ٶȿ졣
��1����ӦA(g)+B(g)
 C(g)+D(g)�����е������仯����ͼ��ʾ���жϸ÷�Ӧ��H 0�����������������
C(g)+D(g)�����е������仯����ͼ��ʾ���жϸ÷�Ӧ��H 0���������������������ȷ��������

��2����Al2O3��Ni������̬���ᷢ�����з�Ӧ��
����(g)=" CO" (g)+ H2O (g)�� ��H1=" +34.0" kJ/mol
����(g)= CO2 (g)+ H2(g) ��H2=" ��7.0" kJ/mol
�����ķ���ʽΪ ���ڸ������£���̬CO2����̬H2��Ӧ������̬CO����̬H2O���Ȼ�ѧ����ʽΪ ��
��3����ͼ��ʾ��ˮ�����Թ�����һö��������������۲죺

I���Թ���Һ����������������Ӧ�� ��
II���Թ���Һ���½������� ��ʴ��
III����Һ��Ϊˮ����Һ��Ϊ��ˮ���������� ����ס����ҡ�����Һ�и�ʴ���ٶȿ졣
��10�֣���1��������1�֣�
��2��CH2O2��1�֣���CO2(g) + H2(g) =" CO(g)" + H2O(g) ��H = +41.0kJ/mol����2�֣�
��3��IO2 + 2H2O + 4e-�� 4OH- ����2�֣�II���⡣��2�֣�III�ҡ���2�֣�
��2��CH2O2��1�֣���CO2(g) + H2(g) =" CO(g)" + H2O(g) ��H = +41.0kJ/mol����2�֣�
��3��IO2 + 2H2O + 4e-�� 4OH- ����2�֣�II���⡣��2�֣�III�ҡ���2�֣�
��

��ϰ��ϵ�д�
�����Ŀ
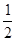 O2(g) �� H2O(g)����H1��a kJ��mol-1?
O2(g) �� H2O(g)����H1��a kJ��mol-1? 2NH3(g) ��H��a kJ��mol��1���Ը��ݱ������м������ݹ���a ��ֵ�� (д�� + ��)��
2NH3(g) ��H��a kJ��mol��1���Ը��ݱ������м������ݹ���a ��ֵ�� (д�� + ��)�� ��g��+1/2O
��g��+1/2O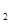 ��g����CO��g��+2H
��g����CO��g��+2H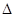 H1����35.6kJ��mol
H1����35.6kJ��mol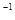

 �����ʵ���Ũ�� ��
�����ʵ���Ũ�� ��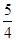 O2��g����
O2��g����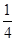 P4O10��s�� ��H����738.5kJ��mol��1
P4O10��s�� ��H����738.5kJ��mol��1 
 (g)+3H
(g)+3H (g) ��H= ��92.4kJ/mol������� 1 mol N��N����Ҫ���յ�����Ϊ�� ��
(g) ��H= ��92.4kJ/mol������� 1 mol N��N����Ҫ���յ�����Ϊ�� ��