��Ŀ����
��8�֣���1����ѧ��Ӧ����Ϊ�ɼ����Ѻ��¼��γɵĹ��̡���ѧ���ļ������γɣ����1 mol��ѧ��ʱ�ͷţ������գ�����������֪��N��N���ļ�����948.9kJ��mol��1��H��H���ļ�����436.0 kJ��mol��1����N2��H2�ϳ�1molNH3ʱ�ɷų�46.2kJ��������N��H���ļ����� �� ��
��2����˹�����������Ϳ�ѧ�о����к���Ҫ�����塣��Щ��Ӧ�ķ�Ӧ����Ȼ��ֱ�Ӳ�ã�����ͨ����ӵķ����ⶨ���ָ�������3���Ȼ�ѧ��Ӧ����ʽ��
Fe2O3(s)+3CO(g)=2Fe(s)+3CO2(g) ��H����24.8 kJ��mol��1
3Fe2O3(s)+ CO(g)==2Fe3O4(s)+ CO2(g) ��H����47.2 kJ��mol��1
Fe3O4(s)+CO(g)==3FeO(s)+CO2(g) ��H����640.5 kJ��mol��1
д��CO���廹ԭFeO����õ�Fe �����CO2������Ȼ�ѧ��Ӧ����ʽ��
_________________ ��
��2����˹�����������Ϳ�ѧ�о����к���Ҫ�����塣��Щ��Ӧ�ķ�Ӧ����Ȼ��ֱ�Ӳ�ã�����ͨ����ӵķ����ⶨ���ָ�������3���Ȼ�ѧ��Ӧ����ʽ��
Fe2O3(s)+3CO(g)=2Fe(s)+3CO2(g) ��H����24.8 kJ��mol��1
3Fe2O3(s)+ CO(g)==2Fe3O4(s)+ CO2(g) ��H����47.2 kJ��mol��1
Fe3O4(s)+CO(g)==3FeO(s)+CO2(g) ��H����640.5 kJ��mol��1
д��CO���廹ԭFeO����õ�Fe �����CO2������Ȼ�ѧ��Ӧ����ʽ��
_________________ ��
��1��391.6 kJ��mol��1 ��2��CO(g)+FeO(s)= Fe(s) + CO2(g) ��H=��218.00kJ��mol��1
���鷴Ӧ�ȵļ��㼰�Ȼ�ѧ����ʽ����д��
��1����Ӧ�Ⱦ��Ƕϼ����յ��������γɻ�ѧ�����ų��������IJ�ֵ����˸��ݺϳɰ��ķ���ʽ��֪��H��948.9kJ/mol��3��436.0 kJ/mol��2��3��x����92.4 kJ/mol�����x��391.6 kJ/mol.
��2�����ݢ�Fe2O3(s)+3CO(g)=2Fe(s)+3CO2(g) ��H����24.8 kJ/mol����3Fe2O3(s)+ CO(g)==2Fe3O4(s)+ CO2(g) ��H����47.2 kJ/mol�͢�Fe3O4(s)+CO(g)==3FeO(s)+CO2(g) ��H����640.5 kJ/mol��֪���١�3���ڣ��ۡ�2�ɵ�6FeO(s)+6CO(g)=6Fe(s)+6CO2(g)�����Է�Ӧ�ķ�Ӧ��Ϊ��24.8 kJ/mol��3��47.2 kJ/mol��640.5 kJ/mol��2����1308.2 kJ/mol���Ȼ�ѧ����ʽΪCO(g)+FeO(s)= Fe(s) + CO2(g) ��H=��218.00 kJ/mol��
��1����Ӧ�Ⱦ��Ƕϼ����յ��������γɻ�ѧ�����ų��������IJ�ֵ����˸��ݺϳɰ��ķ���ʽ��֪��H��948.9kJ/mol��3��436.0 kJ/mol��2��3��x����92.4 kJ/mol�����x��391.6 kJ/mol.
��2�����ݢ�Fe2O3(s)+3CO(g)=2Fe(s)+3CO2(g) ��H����24.8 kJ/mol����3Fe2O3(s)+ CO(g)==2Fe3O4(s)+ CO2(g) ��H����47.2 kJ/mol�͢�Fe3O4(s)+CO(g)==3FeO(s)+CO2(g) ��H����640.5 kJ/mol��֪���١�3���ڣ��ۡ�2�ɵ�6FeO(s)+6CO(g)=6Fe(s)+6CO2(g)�����Է�Ӧ�ķ�Ӧ��Ϊ��24.8 kJ/mol��3��47.2 kJ/mol��640.5 kJ/mol��2����1308.2 kJ/mol���Ȼ�ѧ����ʽΪCO(g)+FeO(s)= Fe(s) + CO2(g) ��H=��218.00 kJ/mol��

��ϰ��ϵ�д�
 ��ʦ�㾦�ִʾ��ƪϵ�д�
��ʦ�㾦�ִʾ��ƪϵ�д�
�����Ŀ

 ��g��+1/2O
��g��+1/2O ��g����CO��g��+2H
��g����CO��g��+2H H1����35.6kJ��mol
H1����35.6kJ��mol
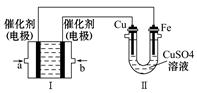
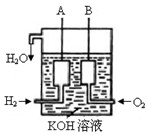
 �����ʵ���Ũ�� ��
�����ʵ���Ũ�� �� 2SO3(g)����H =��Q1kJ/mol������ͬ�¶��£����ܱ�������ͨ��4molSO2��1molO2���ﵽƽ��ʱ�ų�����Q2 kJ�������й�ϵʽ��ȷ����
2SO3(g)����H =��Q1kJ/mol������ͬ�¶��£����ܱ�������ͨ��4molSO2��1molO2���ﵽƽ��ʱ�ų�����Q2 kJ�������й�ϵʽ��ȷ����