��Ŀ����
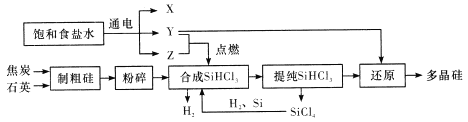
����Ŀ�������ũҵ�����벻�����ʣ��������еĵ��ʶ���Ϊԭ�ϣ�ij��ѧ��ȤС������ͼһװ���Ʊ�������̽��������ʡ�
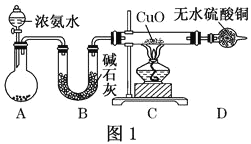
��1��װ��A�У�ʢ��Ũ��ˮ����������Ϊ_____��װ��B��������______��
��2�����Ӻ�װ�ò�����װ�õ������Ժ�װ��ҩƷ��Ȼ��Ӧ��_____����I���
�����������Բ����ƿ�м��백ˮ
����װ��C
��3��ʵ���й۲쵽C��CuO��ĩ��죬D����ˮ����ͭ���������ռ���һ�ֵ������壬��÷�Ӧ��ػ�ѧ����ʽΪ______���÷�Ӧ֤���������л�ԭ�ԣ����������ڴ��������µķ�ӦҲ��������һ���ʣ��÷�Ӧ��ѧ����ʽΪ______��
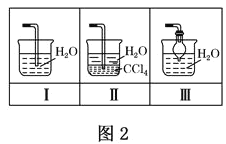
��4����ʵ��ȱ��β������װ�ã�ͼ��������������β����װ����_____����װ����ţ���
��5��ʵ���һ�����ͼ����ʾװ���Ʊ���������ѧ��Ӧ����ʽΪ_____��
��6����3.2gͭ��60.0mLһ��Ũ�ȵ����ᷢ����Ӧ��ͭ��ȫ�ܽ⣬����NO2��NO�����������Ϊ 8.96L(���)��������������ȫ���ͷź�����Һ���� 100mL2.0mol/L �� NaOH��Һ��ǡ��ʹ��Һ�е�Cu2+ȫ��ת���ɳ�������ԭ������Һ��Ũ��Ϊ _______________mol/L��
���𰸡���Һ©�� ���ﰱ�� I 3CuO+2NH3![]() 3Cu+N2+3H2O 4NH3+5O2
3Cu+N2+3H2O 4NH3+5O2![]() 4NO+6H2O �� 2NH4Cl+Ca(OH)2
4NO+6H2O �� 2NH4Cl+Ca(OH)2![]() CaCl2+2NH3��+2H2O 10
CaCl2+2NH3��+2H2O 10
��������
Ũ��ˮ�ڼ�ʯ�һ���ʯ�ҵ������»ӷ����ɰ�����B���ü�ʯ�Ҹ����C�м���������ͭ����������ԭ��Ӧ����ˮ��������ͭ��D�����ڼ����Ƿ�����ˮ���������ˮ���գ�������������ˮ��ע���ֹ������
��1��װ��A�У�ʢ��Ũ��ˮ����������Ϊ��Һ©���������Ǽ������壬�ü��Ը�������Bװ���еļ�ʯ��������ˮ���������ﰱ�������ã��ʴ�Ϊ����Һ©�������ﰱ����
��2�����Ӻ�װ�ò�����װ�õ������Ժ�װ��ҩƷ�������������Բ����ƿ�м���Ũ��ˮ������Ӧ���ɰ������ʴ�Ϊ��I��
��3��ʵ���й۲쵽C��CuO��ĩ���֤������ͭ��D����ˮ����ͭ��������ˮ�����ռ���һ�ֵ������壬����������ԭ��Ӧ����������ͭ��������Ϊ����������ͭ����ԭΪͭ���䷴Ӧ�Ļ�ѧ����ʽΪ��3CuO+2NH3![]() 3Cu+N2+3H2O�������������ڴ��������·�Ӧ����һ��������ˮ���仯ѧ��Ӧ����ʽΪ��4NH3+5O2
3Cu+N2+3H2O�������������ڴ��������·�Ӧ����һ��������ˮ���仯ѧ��Ӧ����ʽΪ��4NH3+5O2![]() 4NO+6H2O���ʴ�Ϊ��3CuO+2NH3
4NO+6H2O���ʴ�Ϊ��3CuO+2NH3![]() 3Cu+N2+3H2O��4NH3+5O2
3Cu+N2+3H2O��4NH3+5O2![]() 4NO+6H2O��
4NO+6H2O��
��4��������������ˮ��β��������Ҫ��ֹ������ͼ2������������β����װ���Ǣ�װ�â������������ʴ�Ϊ����
��5��ʵ�������Ȼ�粒�����������ƹ����Ʊ��������仯ѧ��Ӧ����ʽΪ��2NH4Cl+Ca(OH)2![]() CaCl2+2NH3��+2H2O���ʴ�Ϊ��2NH4Cl+Ca(OH)2
CaCl2+2NH3��+2H2O���ʴ�Ϊ��2NH4Cl+Ca(OH)2![]() CaCl2+2NH3��+2H2O��
CaCl2+2NH3��+2H2O��
��6��ԭ������Һ��HNO3�����ʵ������ڷ�Ӧ����Һ��NO3-��δ����ԭ��HNO3���ͻ�ԭ����NO2��NO�����ʵ����ܺͣ�����ԭ��HNO3������Ӧ����Һ�е�NO3-��������������ȫ���ͷź�����Һ���� 100mL2.0mol/L �� NaOH��Һ��ǡ��ʹ��Һ�е�Cu2+ȫ��ת���ɳ�������ʱ����ΪNaNO3����n(NO3-)=n(Na)=0.1L��2.0mol/L=0.2mol������ԭ��HNO3����ԭ����NO2��NO�����ʵ�������![]() ����ԭ������Һ��Ũ��Ϊ
����ԭ������Һ��Ũ��Ϊ![]() ���ʴ�Ϊ��10��
���ʴ�Ϊ��10��

����Ŀ������������(H2O2)�ǵ���ɫ����Һ�壬����ˮ������Ȼ�ϣ�ˮ��Һ�׳�˫��ˮ��Ϊ��ɫ��Һ�塣ʵ���ҳ��ù���������ȡ��������ҵ�Ϲ�����������Ҫ���������ͻ�ԭ����������������ɱ����Ư�ȡ�ij��ѧ��ȤС���ͬѧΧ�ƹ������չ�˵����о���ʵ�飬�����������һ���������ѧϰ����
(1)������������Ԫ�صĻ��ϼ���__��
(2)ʵ�������ù���������ȡ�����Ļ�ѧ����ʽΪ__��
(3)ͬѧ����0.1000mol��L-1�����Ը�����ر���Һ�ζ�ij�����й�������ĺ�������Ӧԭ��Ϊ2MnO![]() +5H2O2+6H+=2Mn2++8H2O+5O2����
+5H2O2+6H+=2Mn2++8H2O+5O2����
���ڸ÷�Ӧ�У�H2O2��__(����������������ԭ��)�����Ը��������ҺӦװ��__(������ʽ��������ʽ��)�ζ����С�
�����������һ�����Ը�����ر���Һ��__���ζ������յ㡣
������Һ����ȡ25.00mL����������ƿ�У��ظ��ζ��ĴΣ�ÿ�����ĵ�KMnO4����Һ������±���ʾ��
��һ�� | �ڶ��� | ������ | ���Ĵ� | |
���(mL) | 17.10 | 18.10 | 18.00 | 17.90 |
�������й��������Ũ��Ϊ___mol��L-1��
�����ζ�ǰ�����������ݣ��ζ�����ʧ����ⶨ���__(����ƫ��������ƫ��������������)��
(4)ͬѧ�Ƿ�����μ��˷�̪��NaOH��Һ�м���H2O2����Һ�к�ɫ��ʧ��������ɫԭ��ͬѧ��ΪH2O2�Ƕ�Ԫ���ᣬ������OH-ʹ��ɫ��ʧ����ͬѧ��ΪH2O2����Ư����ʹ��Һ��ɫ�������һ����ʵ�鷽�����жϼ�����λͬѧ��˵���Ƿ���ȷ��__��
����Ŀ���±���Ԫ�����ڱ���һ���֣���Ա��еĢ١�����Ԫ�أ����û�ѧ����ش��������⣺
�� ���� | IA | IIA | IIIA | IVA | VA | VIA | VIIA | 0 |
2 | �� | �� | �� | |||||
3 | �� | �� | �� | �� | �� | |||
4 | �� | �� |
��1���ڢۡ���Ԫ���У�ԭ�Ӱ뾶������__________����Ԫ�ط��ţ���
��2����Ԫ�ص�����������Ӧ��ˮ���������⻯����������M��M�к��еĻ�ѧ��������__________________��
��3��д��Ԫ�آٺ͢�ĵ����ڼ��������·�Ӧ���ɵĻ�����ĵ���ʽ��_________��
��4
��5���١�����Ԫ������������Ӧ��ˮ������������ǿ����_____________�������ʻ�ѧʽ���������Ե�����������_________�������ʻ�ѧʽ��,�û�������NaOH��Һ��Ӧ�����ӷ���ʽΪ___________��
��6���õ���ʽ��ʾԪ�آ�����γɻ�����Ĺ���_____________________________��