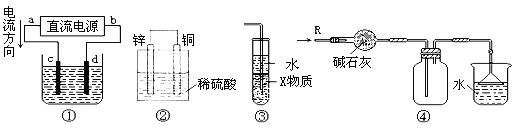
��Ŀ����
����Ŀ���ྦྷ���ǵ��ʹ��һ����̬�����������Ƭ��̫���ܵ�ؼ��ߴ����ƾ�����Ҫԭ�ϡ���֪�ྦྷ���������ҵ��ȡ������ͼ��ʾ��
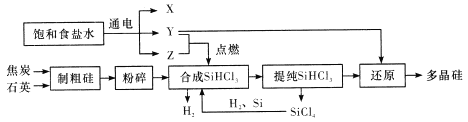
����˵���������
A. Y��Z�ֱ�ΪH2��Cl2
B. ��ȡ�ֹ�Ĺ����н�̿��ʯӢ�ᷢ������Ӧ����̼���裬�ڸø���Ӧ�У��������뻹ԭ�������ʵ���֮��Ϊ1��1
C. SiHCl3����ˮ�⣬����ȫˮ��IJ���ΪH2SiO3��H2��HCl���ݴ��Ʋ�SiHCl3�й�Ԫ�صĻ��ϼ�Ϊ+2��
D. Y��SiHCl3�Ʊ��ྦྷ��ķ�Ӧ�����û���Ӧ
���𰸡�B
��������A. ��ⱥ��ʳ��ˮ�����������ơ�������������Y��Z��ȼ���ϣ�����Y�����뽫����仯�����л�ԭ��������˵��Y��Z�ֱ�ΪH2��Cl2��A��ȷ��B. ��ȡ�ֹ�Ĺ����н�̿��ʯӢ�ᷢ������Ӧ����̼������3C+SiO2![]() SiC+2CO�����ڸø���Ӧ��̼������������Ҳ�ǻ�ԭ�����������뻹ԭ�������ʵ���֮��Ϊ1��2��B������C. SiHCl3��H�ǣ�1����Cl�ǣ�1�������Ԫ�صĻ��ϼ�Ϊ+2����C��ȷ��D. ������SiHCl3�Ʊ��ྦྷ��ķ�Ӧ��SiHCl3+H2��Si+3HCl�������û���Ӧ��D��ȷ����ѡB��
SiC+2CO�����ڸø���Ӧ��̼������������Ҳ�ǻ�ԭ�����������뻹ԭ�������ʵ���֮��Ϊ1��2��B������C. SiHCl3��H�ǣ�1����Cl�ǣ�1�������Ԫ�صĻ��ϼ�Ϊ+2����C��ȷ��D. ������SiHCl3�Ʊ��ྦྷ��ķ�Ӧ��SiHCl3+H2��Si+3HCl�������û���Ӧ��D��ȷ����ѡB��

��ϰ��ϵ�д�
�����Ŀ