��Ŀ����
10����ѧ��ѧʵ�飬����ɫ��pH��ֽ�����ڲⶨ��Һ������ԣ��� 25�棬����Һ��pH=7����ֽ����ɫ����pH��7����ֽ���ɫ����pH��7����ֽ����ɫ����Ҫ��ȷ�ⶨ��Һ��pH������pH�ƣ�pH����Ҫͨ���ⶨ��Һ��H+Ũ�����ⶨ��Һ��pH����1����֪ˮ�д�������ƽ��H2O?H++OH-��H��0������ʹƽ�������ƶ�����������Һ�����ԣ�ѡ��ķ�����C����ĸ����
A����ˮ�м���NaHSO4��Һ
B����ˮ�м���Cu��OH��2����
C������ˮ��100��[����c��H+��=1��10-6 mol•L-1]
D����ˮ�м���H2SO4��Һ
��2�������ⶨ100���ˮ��pH������ԣ�����pH��ֽ�ⶨ������ֽ�Ե���ɫ������pH�Ʋⶨ����pH��7 ���������������=��������Һ�����ԣ���ᡱ������С�����
��3��ij�жԴ������м�⣬���ָ�����Ҫ��Ⱦ��Ϊ�����������PM2.5��ֱ��С�ڵ���2.5 ��m�����������������Ҫ��ԴΪȼú��������β���ȣ���ˣ���PM2.5��SO2��NOx�Ƚ����о�������Ҫ���壮����ø���������ˮ���������ӵĻ�ѧ��ּ���ƽ��Ũ�����±���
| ���� | K+ | Na+ | NH4+ | SO42- | NO3- | Cl- |
| $\frac{Ũ��}{��mol•{L}^{_1}��}$ | 4��10-6 | 6��10-6 | 2��10-5 | 4��10-5 | 3��10-5 | 2��10-5 |
��4������֪����������NO�ķ�ӦΪN2��g��+O2��g��?2NO��g����H��0�����������������¶�Խ�ߣ���λʱ����NO�ŷ���Խ��ԭ�������ȷ�Ӧ���¶����ߣ�ƽ�������ƶ���NO�ĺ�������
������ȼ�Ͳ���ȫȼ��ʱ����CO������������з�Ӧ��ȥCO��2CO��g��=2C��s��+O2��g������֪�÷�Ӧ�ġ�H��0�������������ܷ�ʵ�ֵ����ݡ�H��0��S��0�������κ��¶��¡�G��0���������Է����У�
���� ��1��ʹˮ�ĵ���ƽ�������ƶ��ķ����У������¶ȡ����뺬���������ӵ��εȣ������Һ�����ԣ�˵����������ʺ����������ӷ�Ӧ��������Һ������������Ũ��С��������Ũ�ȶ�ʹ��Һ�����ԣ�
��2��100���ˮ��ʾ���ԣ��������Ի�����pH��ֽ����ɫ��ȷ�����ɣ���25��ʱ��ˮ��pH=7���¶����ߣ���pH����ˮ���κ��¶��¾�Ϊ���ԣ�
��3���ȸ�����Һ�е���غ���������ӵ�Ũ�ȣ�Ȼ�����pH��
��4���ټ����ƽ��ʱ�������ʵ����ʵ��������ƽ�ⳣ���ı���ʽ���㣻
�ڸ���G=��H-T•��S�жϷ�Ӧ�ܷ��Է����У�
��� �⣺��1��A����ˮ�м���NaHSO4��NaHSO4����������ӣ�����ˮ���룬ƽ�������ƶ���������Һ��C��H+����C��OH-������Һ�����ԣ���A����
B����ˮ�м���Cu��NO3��2��Cu��NO3��2��ǿ����������ˮ�⣬ͭ���Ӻ����������ӽ������������ͭ���Ӷ��ٽ�ˮ���룬������Һ��C��OH-����C��H+������Һ�����ԣ���B����
C��ˮ�ĵ��������ȷ�Ӧ��������100�棬�ٽ�ˮ���룬��ҺC��OH-��=C��H+������Һ�����ԣ���C��ȷ��
D����ˮ�м���H2SO4��H2SO4��ǿ�ᣬ�����������������ˮ�ĵ��룬������Һ��C��OH-����C��H+������Һ�����ԣ���D����
��ѡC��
��2���¶�����ٽ�ˮ�ĵ��룬����ˮ��pH���С������100���ˮ��Ȼ�����Եģ�����ʱ��pHֵС��7��pH��ֽ�ⶨ��Һ�������ʱ����ֽΪ���ƣ�ˮ���κ��¶��¾�Ϊ���Եģ��ʴ�Ϊ�����ƣ������У�
��3���۲�����з���NH4+ˮ�������ԣ�PM2.5�������Ϊ���ԣ�������pHֵ������Һ�е���غ㣺c��H+��+c��K+��+c��Na+��+c��NH4+��=2c��SO42-��+c��NO3-��+c��Cl-������H+����Ũ��Ϊ10-4����������pH=4��
�ʴ�Ϊ�����ԣ�4��
��4���������¶�Խ�ߣ���λʱ����NO�ŷ���Խ��ԭ�����¶����ߣ���Ӧ���ʼӿ죬ƽ�����ƣ�
�ʴ�Ϊ�����ȷ�Ӧ���¶����ߣ�ƽ�������ƶ���NO�ĺ�������
��2CO��g��=2C��s��+O2��g�����÷�Ӧ���������ؼ��ķ�Ӧ������G=��H-T•��S��G��0������ʵ�֣�
�ʴ�Ϊ����H��0��S��0�������κ��¶��¡�G��0���������Է����У�
���� �����ۺϿ��黯ѧ��Ӧԭ���Ļ���֪ʶ���漰���ӵ�ˮ�⡢pHֵ�ļ��㡢��ѧƽ�ⳣ���ļ��㡢�����ܵ�Ӧ�õȣ���Ŀ�Ѷ��еȣ�ע�����֪ʶ�Ļ��ۣ�

 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�| A�� |  ������ƿ��ת����Һ | B�� |  �к��ȵIJⶨ | ||
| C�� | 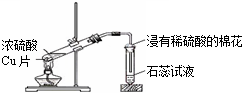 Ũ������ͭ�ķ�Ӧ | D�� |  �Ҷ��ᾧ�����ȷֽ� |
| A�� | ���Ƶ���ˮ�ڹ�����������ɫ��dz | |
| B�� | ��ѹ�Ժϳɰ����� | |
| C�� | ��NO2��N2O4�Ļ�����彵�º�������ɫ��dz | |
| D�� | ���������ʹ�ϳɰ������ʼӿ� |
| A�� | ��ʹ������Ȼ�̼��Һ��ɫ | B�� | ����Ȼ������Ҫ�ɷ� | ||
| C�� | ���ܷ���ȼ�շ�Ӧ | D�� | �ܷ����ӳɷ�Ӧ |
| A�� | N2��O2��� | B�� | ���¡���ѹ�£�ʯīת��Ϊ���ʯ | ||
| C�� | H2��O2��������H2O | D�� | C+O2$\frac{\underline{\;��ȼ\;}}{\;}$CO2 |
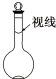 ʵ��������500mL 0.2mol��L-1Na2S04��Һ��ʵ������������£�
ʵ��������500mL 0.2mol��L-1Na2S04��Һ��ʵ������������£�