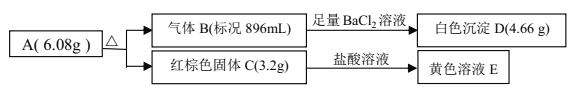
��Ŀ����
����Ŀ����Ũ��SO2�����Ĵ����ǹ�ҵ���⣬��ҵ�ϳ����÷ϼ�������Ҫ�ɷ�Na2CO3���������᳧β���е�SO2�Ʊ���ˮNa2SO3�ijɱ��ͣ��������ԣ����������¡�
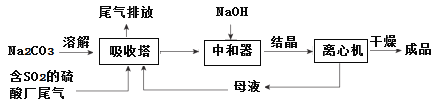
��1��Ϊ��ʹSO2������������ȫ���ڲ��ı�����������������£����Բ�ȡ�ĺ�
����ʩ______________��_______________����д��������
��2���к����з�������Ҫ��Ӧ�����ӷ���ʽ��_____________________��
��3����ͼΪ��������Na2CO3��Һ��SO2��Ӧ��������Һ��ɱ仯��

������ڷ�Ӧ��ͼ��A����ǰ���Ļ�ѧ����ʽ��__________________��
��ͨ����ⷨ�ɷ���ͼ��B��NaHSO3��Na2SO3����ʵ��Na2SO3��ѭ�����ã�ʾ��ͼ���£�
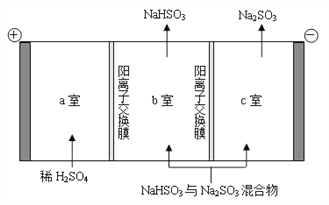
��������NaHSO3��Na2SO3������ԭ��___________________��
��4����ͼ���������Ƶ��ܽ�����ߣ��¶���33��ǰ���Ӧ��ͬ���ʣ�������˵����ȷ����______
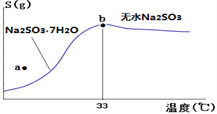
A��a��ʱ��ҺΪ��������Һ
B��b��ʱNa2SO3��7H2O����ˮNa2SO3����
C���Ʊ���ˮNa2SO3��Ӧ����95~100�����Ũ������ȴ�����½ᾧ
��5������ú������ʵ������ʵ����и���Һ�ֱ�����SO2��������������������__________
A��Na2SO3 B��Na2S C��Ba(NO3)2 D������KMnO4
���𰸡�����SO2��������Na2CO3��Һ��Ũ�ȣ��ʵ����¡�������Һ�����������ܷ���Na2CO3��Һ�����Ⱥ������ɣ�HSO3- + OH- = SO32- + H2O 2Na2CO3 + SO2 + H2O =2 NaHCO3 + Na2SO3����4OH��-4e��=2H2O+O2����c(H+)����H����a�Ҿ������ӽ���Ĥ����b�ң�H����SO32�� �������HSO3����Na2SO3ת��ΪNaHSO3������2H��-2e��=H2��������HSO3��![]() H��+ SO32�������ƶ���Na����b�ҽ���c�ң�NaHSO3ת��ΪNa2SO3��BC
H��+ SO32�������ƶ���Na����b�ҽ���c�ң�NaHSO3ת��ΪNa2SO3��BC
����������1���ô�����Һ���պ���SO2��β��������ͨ������SO2���١�����Na2CO3��Һ��Ũ�ȡ�������Һ�����������ܷ���Na2CO3��Һ�����ȴ�ʩʹSO2������������ȫ��
��2�����Ŷ�������ͨ����������������Ҫ�ǵõ����������ƣ��к�������Ҫ�ǽ�����������ת��Ϊ�����ƣ�ͬʱ̼�����Ʒ�Ӧ�õ�̼���ƣ���Ҫ��Ӧ��ѧ����ʽΪ��NaHSO3+NaOH=Na2SO3+H2O����Ӧ�����ӷ���ʽΪHSO3- + OH- = SO32- + H2O��
��3������ͼ��֪�����ڷ�Ӧ��ͼ��A����ǰ��̼�������������Ӧ����̼���������������ƣ���Ӧ����ʽΪ2Na2CO3 + SO2 + H2O =2 NaHCO3 + Na2SO3 -��
��ͨ����ⷨ�ɷ���NaHSO3��Na2SO3��������2H2O-4e-=4H++O2����c��H+������H+��a�Ҿ������ӽ���Ĥ����b�ң�H+��SO32-�������HSO3-��Na2SO3ת��ΪNaHSO3������2H+-2e-=H2��������HSO3-![]() H++SO32-�����ƶ���Na+��b�ҽ���c�ң�NaHSO3ת��ΪNa2SO3��ʵ��Na2SO3��ѭ�����ã�
H++SO32-�����ƶ���Na+��b�ҽ���c�ң�NaHSO3ת��ΪNa2SO3��ʵ��Na2SO3��ѭ�����ã�
��4��A��a�����ܽ�������Ϸ�����ʱ��ҺΪ��������Һ���ڶ�Ӧ�¶��»��о�����������A����B����b�������ľ��������Na2SO3��7H2O����ˮNa2SO3�Ļ������߹��棬��B��ȷ�� C�����Ʊ���ˮNa2SO3��Ӧ����95~100������Ũ�����ᾧ�������ȹ��ˣ�������ȴ�ᾧ����C����ΪB��
��5������ú������ʵ������ʵ����и���Һ�ֱ�������SO2��
A��Na2SO3 ���ն����������ķ�ӦΪ��Na2SO3+SO2+H2O=2NaHSO3��1molNa2SO3 ������ն�������1mol��B��Na2S���ն����������ķ�ӦΪ��2Na2S+5SO2+2H2O=4NaHSO3+3S����1mol2Na2S��෴Ӧ��������2.5mol��C��Ba��NO3��2���ն�������Ӧ��ѧ����ʽΪBa��NO3��2+3SO2+2H2O=BaSO4��+2H2SO4+2NO����1molBa��NO3��2������ն�������3mol��D������KMnO4��Һ���ն�������ķ�Ӧ2MnO4-+5SO2+2H2O=2Mn2++5SO42-+4H+��1molKMnO4��෴Ӧ��������2.5mol����ΪC��

����Ŀ��ij��Һ�У�������ˮ�ĵ��룬ֻ�����±�����ʾ���������ӣ�.�����X�������༰�����bΪ
�������� | Na+ | Al3+ | Cl- | X |
���� | 2a | a | a | b |
A. NH4+��4a B. SO42-��2a C. OH-��4a D. CO32-��2a
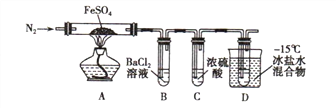
����Ŀ��ʵ������ijЩ�������ȡ���ռ���β������װ����ͼ��ʾ(ʡ�Լгֺ;���װ��)�����ô�װ�úͱ����ṩ������������ʵ�飬�������ѡ����

ѡ�� | a�е����� | b�е����� | c���ռ������� | d�е����� |
A | Ũ��ˮ | CaO | NH3 | H2O |
B | Ũ���� | Na2SO3 | SO2 | NaOH��Һ |
C | ϡ���� | Cu | NO2 | H2O |
D | Ũ���� | MnO2 | Cl2 | NaOH��Һ |
A. A B. B C. C D. D
����Ŀ���±��е��������ƻ�1mol�����еĻ�ѧ�������ĵ�������kJ����
���� | Cl2 | Br2 | I2 | HCl | HBr | HI | H2 |
������kJ�� | 243 | 193 | 151 | 432 | 366 | 298 | 436 |
�����������ݻش��������⣺
��1���������ʱ������е�������͵��� ��
A��H2 B��Cl2C��Br2D��I2
��2��X2+H2![]() 2HX (X����Cl��Br��I)�ķ�Ӧ�Ƿ��ȷ�Ӧ�������ȷ�Ӧ��
2HX (X����Cl��Br��I)�ķ�Ӧ�Ƿ��ȷ�Ӧ�������ȷ�Ӧ��
��3����ͬ�����£�X2(X����Cl��Br��I)�ֱ���������Ӧ�������ĵ����ʵ���������ʱ���ų������յ����������� ��
��4�������ϱ��е����ݣ����ܻش����⣨3���� ���ش������������������������������ ��