��Ŀ����
����Ŀ���Ѱۣ����ʯ���� TiO2�����㷺�����������ɫ���ᡣ��ҵ������������Ҫ�ɷ�Ϊ FeTiO3������ Fe2O3 �� SiO2 �����ʣ�Ϊԭ�����Ѱ۵���Ҫ�������£�

�ش��������⣺
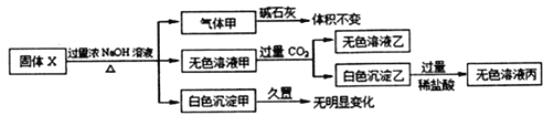
��1���ڢڲ����������ijɷ���________��
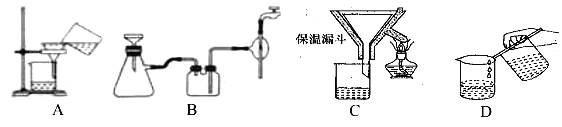
��2������������Ҫ�����������γ� TiO2��nH2O �ܽ����ù��չ�����Ҫ��ʵ��������ɣ����еġ����ˡ���������ѡ������װ��________����ѡ���
��3���ڢ۲���ʵ�������______�����ˣ��Ӷ���ø���Ʒ FeSO4��7H2O��
��4��Ϊ�ⶨ������������Һ��TiO2+��Ũ�ȣ�ȡ������Һ10 mL ������ˮϡ����100 mL������������ۣ������ʹ����ȫ��Ӧ��3TiO2+ + Al + 6H+ = 3Ti3+ + Al3+ + 3H2O�����˺�ȡ����Һ20.00 mL��������ʱ������Һ����ı仯���Բ��ƣ��������еμ�2��3 ��KSCN��Һ��ָʾ������ 0.1000 mol��L-1NH4Fe(SO4)2 ����Һ�ζ�����Һ���ֺ�ɫ����ʱ��Һ�� Ti3+ȫ��������Ϊ Ti4+�����ı�Һ30.00mL���ش��������⣺
�����в�����ʹ���� TiO2+Ũ��ƫ�ߵ���______��
A. �����Ʊ�Һ�Ĺ����У�δϴ���ձ��Ͳ�����
B. �����Ʊ�Һ�����Ǹ��ӿ̶���
C��������ˮϴ�Ӻ�δ����ϴ�ĵζ���ȡ����Һ
D���ڵζ��յ����ʱ���ӵζ��̶ܿ���
����ô�����Һ�� TiO2+�����ʵ���Ũ����______��
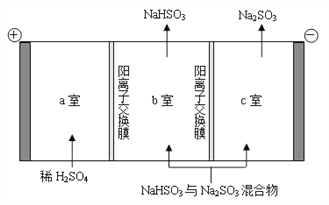
��5����ѧ�Ҵӵ��ұ�����Ĺ����еõ��������ҳ���ұ���ѵ��¹��ա�TiO2 ֱ�ӵ�ⷨ�����ѣ������Ϊ���ڵ��Ȼ��ƣ�ԭ����ͼ��ʾ��

д�������缫��Ӧʽ___________��
���𰸡�Fe��SiO2C����Ũ������ȴ�ᾧAD1.500 mol��L��1TiO2��4e��=Ti��2O2��
��������
(1)�������к��е� SiO2 ���������ᣬ�ӻ��Һ�м����˹�����Fe�ۣ���ԭ��Һ�е�Fe3+������������������ijɷ���SiO2 ������Fe��
(2)����������Ҫ�����¶����γ� TiO2��nH2O �ܽ�����Ӧѡ����©����ɡ����ˡ���������ѡ��C�������⣬��ΪC��
(3)��������������Һͨ������ ����Ũ������ȴ�ᾧ���ٹ��ˣ��ɵõ�FeSO4��7H2O��
(4)��A�������Ʊ�Һ�Ĺ����У�δϴ���ձ��Ͳ���������Һ��Ũ�Ƚ��ͣ����ĵı�Һ������������� TiO2+Ũ��ƫ�ߣ���A��ȷ��B�������Ʊ�Һ�����Ǹ��ӿ̶��ߣ���Һ���ƫС����Һ��Ũ�Ƚ������ĵı�Һ�����С���������� TiO2+Ũ��ƫ�ͣ���B����C��������ˮϴ�Ӻ�δ����ϴ�ĵζ���ȡ����Һ������Һ��ˮϡ�ͣ�Ũ��ƫ�ͣ���C����D���ڵζ��յ����ʱ���ӵζ��̶ܿ��ߣ���Һ�����ƫ�������� TiO2+Ũ��ƫ�ߣ���D��ȷ����ΪAD��
�����ı�Һ�����ʵ���Ϊ 0.1000 mol��L-1��0.03 L=3��10-3 mol�����ݵ����غ㣬��֪��Һ��Ti3+�����ʵ���Ϊ3��10-3 mol������Һ��TiO2+�����ʵ���Ϊ3��10-3 mol����Һ�� TiO2+�����ʵ���Ũ����3��10-3 mol��0.02 L=1.500 mol��L��1��
(5)���ʱ�������ϵõ��ӷ�����ԭ��Ӧ�����Զ������ѵõ��������Ѻ������ӣ��͵�Դ�����������缫��ӦʽΪTiO2+4e-=Ti+2O2-��

����Ŀ����ȥ�������������������ʣ�ѡ�õ��Լ���ȷ����
ѡ�� | ���ʣ����ʣ� | �Լ� |
A | Al2O3(SiO2) | NaOH��Һ |
B | CO2(SO2) | Na2CO3��Һ |
C | NO(NO2) | ˮ |
D | NaHCO3(Na2CO3) | Ca(OH)2��Һ |
A. A B. B C. C D. D