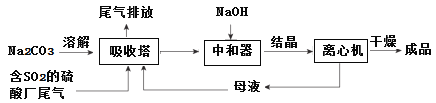
��Ŀ����
����Ŀ����ҵ�ϳ�ͨ�����·ֽ�FeSO4�ķ����Ʊ�Fe2O3��Ϊ����FeSO4���·ֽ�IJ���������й�̽��ʵ�飬�ش��������⣺
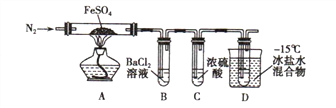
ʵ��һ�����·ֽ�FeSO4��������ͼ��ʾ��ʵ��װ�ý���ʵ�顣
��֪����SO2�۵�Ϊ-72�棬�е�Ϊ-10�� �� SO3�۵�Ϊ-16.8�棬�е�Ϊ44.8��
��1������װ�ã��������������ã�����ҩƷ��ͨ��һ��ʱ��N2Ȼ����ȣ�ͨ��N2��Ŀ����_________________________
��2����������������650�棬����B���а�ɫ������D�Թ�������ɫҺ�壬Ӳ�ʲ������еĹ����Ϊ_________ɫ��д���÷�Ӧ�Ļ�ѧ����ʽ___________________��
��3����Ӧ��Ϻ�ֹͣ������ȴ��ȡӲ�ʲ������й��壬�����ᣬ��Ӧ�����ӷ���ʽ��__________________�� ����Ӧ��������Һ����D�Թ��У���Һ��Ϊdz��ɫ���÷�Ӧ�����ӷ���ʽ�� _______________
ʵ��� ̽�����·ֽ� FeSO4���ɵ�����
��4������ͼ��ʾװ�����ʵ�飬��֤���·ֽ�FeSO4���ɵ���̬����
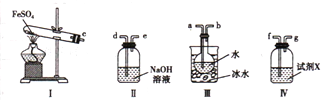
�ٰ������������Ӹ�����������ĸ��ʾ�ӿڵ�����˳��c-__________________________
���Լ�X�������� ___________________________
�۳�ַ�Ӧ������װ��III��Բ����ƿ�ڻ����ⶨ�ѷֽ��FeSO4 ����������Բ����ƿ�������Ȼ�����Һ��ֱ��������ȫ��Ȼ����˻����ڹ������Ͻ�����ϴ����ɲ���ȴ�����£����ء������յõ�����������ΪWg �����ѷֽ��FeSO4������ ________________g��
���𰸡��ų�װ���еĿ�������2FeSO4(����)=Fe2O3+SO2��+SO3��Fe2O3+6H+=2Fe3++3H2O2Fe3+ + SO2 + 2H2O = 2Fe2+ + SO42-+4H+a-b-f-g-dƷ����Һ304W/233
����������1��ͨ��N2��Ŀ�����ų�װ���еĿ�����
��2��B���а�ɫ����������Ϊ���ᱵ���������ᱵ����������ɫ�������ɣ���ӦΪΪ�������������������Ԫ�ػ��ϼ۽��ͣ�����Ԫ�ػ��ϼ�����Ϊ�����������������Ϊ����ɫ��������Ӧ�Ļ�ѧ����ʽΪ2FeSO4![]() Fe2O3+SO2��+SO3����
Fe2O3+SO2��+SO3����
��3��Fe2O3�����ᷴӦ�����ӷ���ʽΪ��Fe2O3+6H+=2Fe3++3H2O������Ӧ��������Һ����D�Թ��У���Һ�����ӱ���ԭΪ��Ϊdz��ɫ���������ӣ�������������Ϊ��������ӣ��÷�Ӧ�����ӷ���ʽ��2Fe3++SO2+2H2O=2Fe2++SO42-+4H+��
��4����Aװ��ΪFeSO4�ڸ����·ֽ�����Fe2O3��SO3��SO2��װ�ã�����������ˮ��Ӧ�������ᣬ���������۵�ߣ������ñ�ˮ�����ʹ��������Һ������c��a�������������������Ʒ����Һ����D��װ��Ʒ����Һ����b��f���������������ж�������Ⱦ��������������������Һ���գ���g��d������
��SO2�������Ư���ԣ���ʹƷ����Һ��ɫ����Dװ����װ��Ʒ����Һ�����������������ɣ�
����һ������Բ����ƿ����μ����Ȼ�����Һ��ֱ��������ȫ���ڶ��������˻����ڹ������Ͻ�����ϴ����ɲ���ȴ�����£����أ���������������ɡ���ȴ������ֱ���������γ��������������0.1gΪֹ�������յõ�����������ΪW g������Ϊ���ᱵ��������Ԫ���غ��ϻ�ѧ����ʽ������ϵ��֪��
2FeSO4��SO3��BaSO4
2 1
n��FeSO4�� ![]()
�ѷֽ��������������Ϊ![]() ��2��152g/mol=
��2��152g/mol=![]() g��
g��

����Ŀ����ȥ�������������������ʣ�ѡ�õ��Լ���ȷ����
ѡ�� | ���ʣ����ʣ� | �Լ� |
A | Al2O3(SiO2) | NaOH��Һ |
B | CO2(SO2) | Na2CO3��Һ |
C | NO(NO2) | ˮ |
D | NaHCO3(Na2CO3) | Ca(OH)2��Һ |
A. A B. B C. C D. D
����Ŀ����ͼ����ʾ��װ�ý���ʵ��,ʵ��������Ԥ����һ������( )
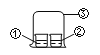
ѡ�� | ���е����� | ���е����� | Ԥ������ |
A | Ũ��ˮ | FeCl3��Һ | �����к��ɫ���� |
B | Ũ��ˮ | Ũ���� | �����а��� |
C | Ũ���� | ����KI��Һ | ������Һ�����Ա仯 |
D | Ũ���� | ��̪��Һ | ������Һ�����Ա仯 |
A. A B. B C. C D. D